Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Grzesik, Zbigniew
Dickerson, Matthew B.
and
Sandhage, Ken H.
2003.
Incongruent reduction of tungsten carbide by a zirconium-copper melt.
Journal of Materials Research,
Vol. 18,
Issue. 9,
p.
2135.
Liu, Che-Ming
Chen, Jyh-Chen
and
Chen, Chun-Jen
2005.
The growth of an epitaxial Mg–Al spinel layer on sapphire by solid-state reactions.
Journal of Crystal Growth,
Vol. 285,
Issue. 1-2,
p.
275.
Liu, Che-Ming
Chen, Jyh-Chen
and
Chen, Chun-Jen
2006.
The morphology of an epitaxial Mg–Al spinel layer on a sapphire surface.
Journal of Crystal Growth,
Vol. 292,
Issue. 2,
p.
302.
Liu, C.M.
Chen, J.C.
Hu, L.J.
and
Lin, S.P.
2006.
The effect of annealing, precipitation-strengthening, and compressive coating processes on sapphire strength.
Materials Science and Engineering: A,
Vol. 420,
Issue. 1-2,
p.
212.
Liu, Yajun
Lipke, David W.
Zhang, Yunshu
and
Sandhage, Kenneth H.
2009.
The kinetics of incongruent reduction of tungsten carbide via reaction with a hafnium–copper melt.
Acta Materialia,
Vol. 57,
Issue. 13,
p.
3924.
Wang, Guigen
Zuo, Hongbo
Zhang, Huayu
Wu, Qibao
Zhang, Mingfu
He, Xiaodong
Hu, Zhaohui
and
Zhu, Lin
2010.
Preparation, quality characterization, service performance evaluation and its modification of sapphire crystal for optical window and dome application.
Materials & Design,
Vol. 31,
Issue. 2,
p.
706.
Wang, Y.
Liu, X.P.
and
Qin, G.W.
2014.
Microstructure of spinel islands on the sapphire surface grown by ion implantation and annealing.
Micron,
Vol. 64,
Issue. ,
p.
6.
Kumamoto, Akihito
Shibata, Naoya
Nayuki, Kei-ichiro
Tohei, Tetsuya
Terasaki, Nobuyuki
Nagatomo, Yoshiyuki
Nagase, Toshiyuki
Akiyama, Kazuhiro
Kuromitsu, Yoshirou
and
Ikuhara, Yuichi
2016.
Atomic structures of a liquid-phase bonded metal/nitride heterointerface.
Scientific Reports,
Vol. 6,
Issue. 1,
Zhang, Yunshu
Cai, Ye
Hwang, SungHwan
Wilk, Gregory
DeAngelis, Freddy
Henry, Asegun
and
Sandhage, Kenneth H.
2018.
Containment materials for liquid tin at 1350 °C as a heat transfer fluid for high temperature concentrated solar power.
Solar Energy,
Vol. 164,
Issue. ,
p.
47.
Chen, Yajin
Zuo, Peng
Guan, Yingxin
Yusuf, M. Humed
Babcock, Susan E.
Kuech, Thomas F.
and
Evans, Paul G.
2020.
Reduction of Interface Reactions in the Low-Temperature Solid-Phase Epitaxy of ScAlMgO4 on Al2O3(0001).
Crystal Growth & Design,
Vol. 20,
Issue. 9,
p.
6001.
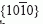 faces of sapphire were exposed to molten magnesium at 1000 °C for 100 h. Such exposure resulted in the formation of a continuous, epitaxial layer of spinel (MgAl2O4) on sapphire and a continuous, epitaxial layer of magnesia (MgO) on the spinel. X-ray pole figure analyses indicated that two variants of spinel and magnesia had formed in a manner consistent with the following orientation relationships:
faces of sapphire were exposed to molten magnesium at 1000 °C for 100 h. Such exposure resulted in the formation of a continuous, epitaxial layer of spinel (MgAl2O4) on sapphire and a continuous, epitaxial layer of magnesia (MgO) on the spinel. X-ray pole figure analyses indicated that two variants of spinel and magnesia had formed in a manner consistent with the following orientation relationships: