Article contents
Doping iodine in CdS for pure hexagonal phase, narrower band gap, and enhanced photocatalytic activity
Published online by Cambridge University Press: 23 February 2011
Abstract
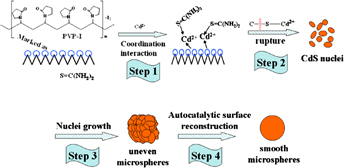
Iodine-doped CdS (I-CdS) with controllable morphologies, pure hexagonal phase, and enhanced photocatalytic activity was synthesized via a mild hydrothermal process with polyvinylpyrrolidone-iodine (PVP-I) acting as the template-directing reagent and iodine source. The morphologies of the as-prepared samples could be adjusted from irregular cone-shaped particles to uneven microspheres, further to smooth microspheres, while the crystal phases were also transformed from mixed cubic and hexagonal phases to pure hexagonal phase upon increasing the molar ratio of PVP-I to Cd2+ from 0 to 2. The iodine doping could result in red shift of the absorption edges and band gap narrowing of the I-CdS samples. Importantly, a critical point of 0.5 of molar ratio of PVP-I to Cd2+ for iodine doping was found to be necessary for obtaining a pure hexagonal phase that facilitates the improving of photocatalytic activity on the degradation of Rhodamine B in aqueous solution under visible light irradiation.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 5
- Cited by




