Article contents
Combustion synthesis and photoelectrochemical characterization of gallium zinc oxynitrides
Published online by Cambridge University Press: 06 November 2018
Abstract
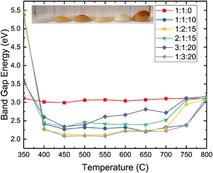
We report a rapid combustion synthesis method for producing band gap tunable gallium zinc oxynitrides, a material of interest for water splitting applications. By varying the ratio of zinc and gallium, we can tune the band gap from 2.22 to 2.8 eV. Furthermore, nitrogen can be incorporated up to nearly 50% via replacement of oxygen without the need for high temperatures or an additional ammonolysis step. X-ray photoelectron spectroscopy (XPS) and EDX analysis suggests a preferential segregation of Zn to the surface of the as-synthesized particles, though the surface Ga/Zn molar ratio in the as-synthesized particles is correlated with the Ga/Zn molar ratio of the precursor materials. Photoelectrochemical measurements show that the oxynitride powders are photoactive under both AM1.5 and visible-only (λ > 435 nm) irradiation. Hydrogen and oxygen were both evolved in half-reaction experiments under simulated AM1.5 irradiation without externally applied bias, although addition of an OER catalyst did not enhance the rate of oxygen formation, suggesting that intra- and interparticle recombination are significant.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 3
- Cited by




