Article contents
Borospherene molecular junction-based sensor for detecting radium and radon in water
Published online by Cambridge University Press: 12 August 2020
Abstract
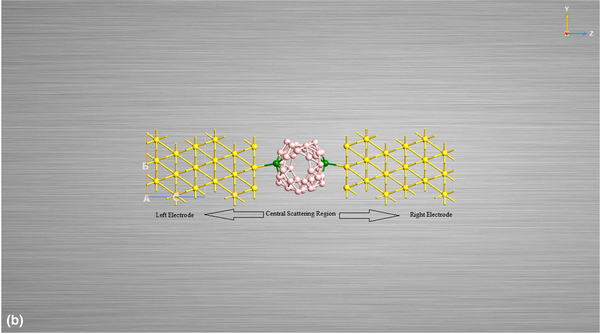
The capability of borospherene to detect radioactive pollutants (radon and radium) is investigated utilizing density functional theory and nonequilibrium Green's function regime. The quantum transport is evaluated by calculating the density of states, chemical potential, transmission and molecular energy spectra, highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap, electron densities, current–voltage curve, and differential and quantum conductance. LUMO-mediated transmission is observed in all the cases. The conduction considerably declines in B40 molecular junction doped with radioactive pollutants in comparison to pure B40 molecular junction. This decrease in conduction is due to reduced electron density and higher chemical potential in doped B40 junctions. Due to different values of current and differential conductance, we propose utilization of B40 in detecting the presence of radioactive pollutants in underground water. Also, all molecular junctions assay lifting of Coulomb blockade at equilibrium state; thus, these devices can be effectively utilized in single-electron transistor applications.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 2
- Cited by





