Article contents
Atomistic kinetic Monte Carlo—Embedded atom method simulation on growth and morphology of Cu–Zn–Sn precursor of Cu2ZnSnS4 solar cells
Published online by Cambridge University Press: 17 January 2020
Abstract
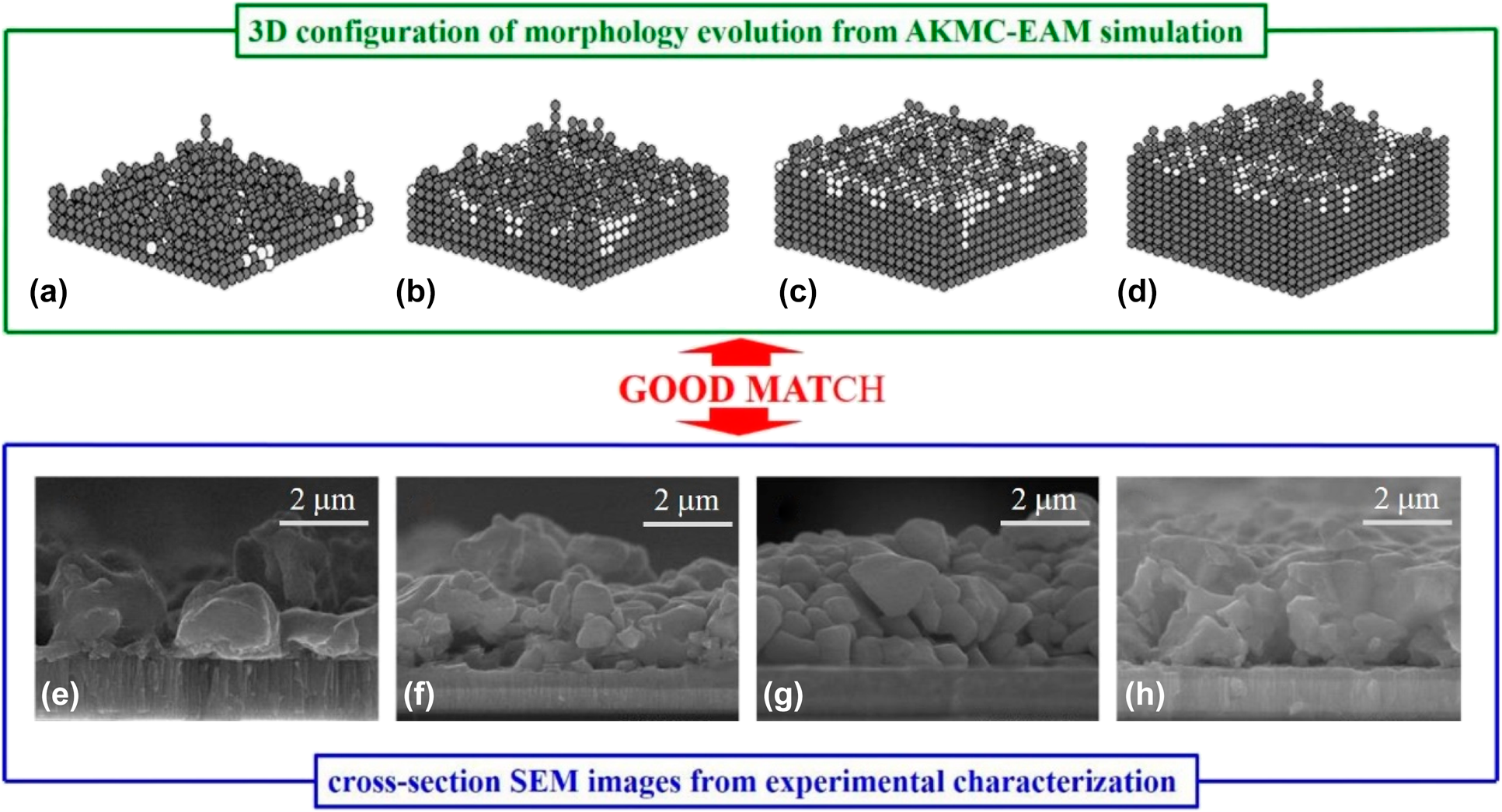
An atomistic kinetic Monte Carlo coupled with the embedded-atom method is used to simulate film growth and morphology evolution of a Cu–Zn–Sn precursor of Cu2ZnSnS4 solar cells by single-step electrodeposition. The deposition and diffusion events of three different metallic atoms are described by the simulation. Moreover, the multibody Cu–Zn–Sn potential is used to calculate diffusion barrier energy. The effects of process factors, including temperature and electrode potential, on the cross-section morphology and surface roughness are explored, while keeping the elemental composition ratios constant. The lowest roughness with the smoothest morphology is obtained at the optimal parameters. The distribution and transformation behaviors of cluster sizes are investigated to describe the alloy film growth process. Furthermore, the comparison between deposition events and diffusion events reveals that deposition events depend primarily on individual deposition rates of different metallic atoms, but diffusion events are mainly dependent on the interaction of metallic atoms. The film morphology evolution is visualized by three-dimensional configuration with increasing numbers of atoms, which suggests a competing mechanism between nucleation and growth of the thin film alloy.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 3
- Cited by




