Article contents
Sorafenib delivered by cancer cell membrane remodels tumor microenvironment to enhances the immunotherapy of mitoxantrone in breast cancer
Published online by Cambridge University Press: 18 November 2020
Abstract
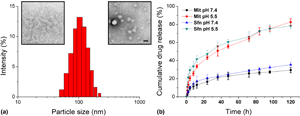
The negative regulation effect of tumor microenvironment (TME) greatly compromised the efficacy of various cancer treatments, especially cancer immunotherapy. As a result, it is generally recognized that remodeling of TME along with the treatment is a promising way to realize satisfactory cancer therapy. Here, in our study, a drug delivery system (DDS) composed cancer cell membrane (CCM) vehicle loaded mitoxantrone (Mit) and sorafenib (Sfn) was proposed with the aim to combine TME regulation and chemotherapy-induced immunotherapy in one platform. Our results confirmed that after treating with this DDS, the Mit induced immunogenic cell death (ICD) could be augmented by Sfn-based TME regulation to realize effective cancer immunotherapy. The Sfn was shown to downregulate of the regulatory T cells (Treg) level while activating the effector T cells of TME. The synergetic TME regulation along with cancer immunotherapy might be a promising way for advanced cancer treatment.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 1
- Cited by





