Article contents
Simple synthesis of polyaniline microtubes for the application on silver microrods preparation
Published online by Cambridge University Press: 05 January 2012
Abstract
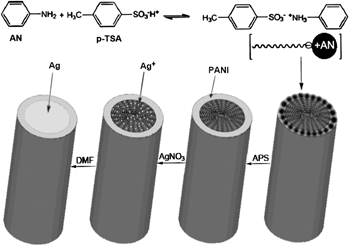
Polyaniline (PANI) microtubes were successfully synthesized by a simple way without using any templates. Their structure was characterized by scanning electron microscopy, transmission electron microscopy, x-ray diffraction, ultraviolet–visible absorption spectra, and Fourier transform infrared spectroscopy. The average length and diameter of the microtubes were about 12.0 and 3.0 μm, respectively. In addition, silver microrods were further prepared using the PANI microtubes as templates. Scanning electron microscopy, energy-dispersive x-ray spectra, x-ray diffraction, and ultraviolet–visible absorption spectra analyses were performed to characterize the structure of the sample. The results indicated the formation of silver microrods inside PANI microtubes. Moreover, the microwave absorption and electrical properties of PANI microtubes, PANI particles, and silver microrods were compared. It shows that the silver microrods coated with PANI have good microwave absorption and electrical properties, which can apply on electromagnetic interference shielding and microwave absorption materials.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 4
- Cited by




