Article contents
Reaction pathways and optoelectronic characterization of single-phase Ag2ZnSnS4 nanoparticles
Published online by Cambridge University Press: 07 November 2019
Abstract
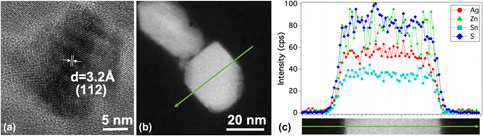
Sputtered thin films of Ag2ZnSnS4 (AZTS) have shown promising semiconducting properties in spite of the films containing SnS2, SnSx, or ZnS as impurity phases. In this study, reaction pathways were identified to produce single-phase AZTS nanoparticles as precursors for forming dense, single-phase films. The morphology, composition, and phase evolution during nanoparticle formation in an oleylamine-based solvothermal reaction process were determined using surface-enhanced Raman spectroscopy (SERS) and transmission and scanning transmission electron microscope (TEM/STEM). The reaction pathways for AZTS nanoparticles were found to be different from Cu2ZnSnS4 nanoparticles in oleylamine, which may explain the difficulty in creating (Ag, Cu)2ZnSnS4 solid solutions in the nanoparticle synthesis. The single-phase AZTS nanoparticle films have a band gap (2.16 eV) slightly higher than sputtered films, and photoelectrochemical (PEC) measurements demonstrated a current of 0.1 mA/cm2 in K2SO4 solution even as porous nanoparticle films, suggesting the potential of this material in solar energy conversion when converted into a dense film.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 8
- Cited by




