Article contents
Preparation and electrochemical evaluation of NiO nanoplatelet-based materials for lithium storage
Published online by Cambridge University Press: 17 July 2014
Abstract
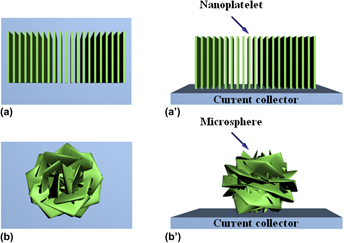
NiO nanoplatelet-based materials with different dimensionality are synthesized by a one-step hydrothermal route at 120 °C for 4 h. The morphologies and structure of the obtained NiO nanoplatelets grown on Ni foam and NiO microspheres composed of nanoplatelets are characterized. The results show that the former has a length of 5–10 μm and a uniform thickness of ∼100 nm, while the latter has a diameter of 5–10 μm. Their electrochemical properties as anode materials for lithium-ion batteries are evaluated and compared. The discharge capacities of NiO nanoplatelet electrode are 663, 516, 370, 258, and 169 mAh g−1 at current densities of 250, 500, 1000, 2500, and 5000 mA g−1, respectively. Such a lithium storage capability is much higher than that of the NiO microsphere electrode. The reasons for the enhanced electrochemical performance of the nanoplatelet electrode were investigated, which suggested that more active sites for electrochemical reactions and faster ion/electron transfer realized on nanoplatelets are facilitating lithium storage.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 5
- Cited by




