Article contents
A novel Fe2O3 rhombohedra/graphene composite as a high stability electrode for lithium-ion batteries
Published online by Cambridge University Press: 02 March 2015
Abstract
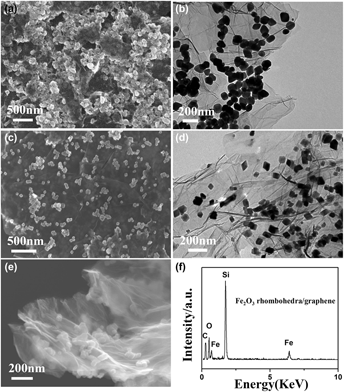
We demonstrate in this paper the shape-controlled synthesis of α-Fe2O3 rhombohedra anchored graphene nanocomposites through a simple hydrothermal strategy by adopting inorganic species in the synthesis system. TEM investigations reveal that the rhombohedra with an average diameter of 80 nm is formed through oriented attachment of primary nanocrystals assisted by Ostwald ripening, and CH3COONa inorganic surfactant played an important role in control over the final morphology of the products. As high-performance anodes for lithium-ion batteries, the obtained Fe2O3 rhombohedra/graphene composite exhibits the first reversible capacity of 905.3 mAh g−1, and high capacity retention of 85.7% after 50 cycles. These values are much higher than those of bare Fe2O3 and Fe2O3 particle/graphene composites, indicating its excellent electrochemical stability. These results give us a guideline for the study of the morphology-dependent properties of functional oxide materials as well as further applications for magnetic materials, lithium-ion batteries, and gas sensors.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 8
- Cited by




