Article contents
Neutron reflectometry analysis of Li4Ti5O12/organic electrolyte interfaces: characterization of surface structure changes and lithium intercalation properties
Published online by Cambridge University Press: 19 September 2016
Abstract
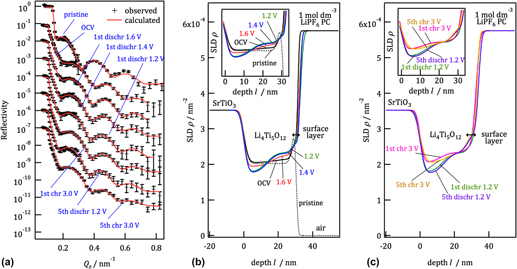
The structure changes and lithium intercalation properties in the surface region of Li4Ti5O12 were investigated using epitaxial Li4Ti5O12(111) film model electrodes. The discharge–charge measurements, which were conducted with 1 mol/dm3 LiPF6-containing propylene carbonate, revealed that a 23.8 nm-thick film exhibited a small capacity of 115 mA h/g compared to the theoretical value of 175 mA h/g. In situ neutron reflectometry and ex situ x-ray diffractometry and reflectometry indicated that an irreversible phase change had occurred in the 10-nm surface region of Li4Ti5O12 during the initial reaction processes. The level of deterioration of the surface structure was significantly reduced by decreasing the LiPF6 concentration; in addition, side reactions of the cell components with the electrolyte species, and their products, may be associated with the deterioration of the Li4Ti5O12 surface. The surface reactions have a significant impact on the capacity of lithium intercalation in nano-sized Li4Ti5O12.
- Type
- Invited Paper
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 9
- Cited by





