Introduction
Chronic rhinosinusitis without nasal polyps is one of the most common chronic inflammatory diseases.Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody1 The current medical treatment options for chronic rhinosinusitis include nasal saline irrigation, intranasal corticosteroids, and antibiotics and/or systemic corticosteroids. Nonetheless, a fraction of patients do not respond optimally to these treatments and require endoscopic sinus surgery.Reference Hastan, Fokkens, Bachert, Newson, Bislimovska and Bockelbrink2 Hence, the most effective treatment for chronic rhinosinusitis has remained a matter of debate.
Recently, long-term, low-dose macrolide therapy has been suggested as an alternative treatment for chronic rhinosinusitis and other respiratory diseases.Reference Cervin and Wallwork3 A growing body of evidence suggests that several macrolides, such as erythromycin, azithromycin and clarithromycin, are effective for the treatment of chronic rhinosinusitis and other respiratory diseases. These macrolides can inhibit or kill bacterial pathogens, and down-regulate pro-inflammatory mechanisms.Reference Wallwork, Coman, Mackay-Sim, Greiff and Cervin4, Reference Cervin, Wallwork, Mackay-Sim, Coman and Greiff5 For example, macrolides demonstrate immunomodulatory capacities by inhibiting cytokines such as interleukin (IL)-8, thereby suppressing neutrophil migration and adhesion, and modulating mucus synthesis and secretion.Reference Cervin and Wallwork3 Several reports,Reference Cervin and Wallwork3–Reference Cervin, Wallwork, Mackay-Sim, Coman and Greiff5 including our previous study,Reference Luo, Chen, Liu, Li, Xu and Fan6 have thus suggested that long-term, low-dose macrolide treatment may be most beneficial in chronic rhinosinusitis patients with a neutrophilic inflammatory pattern and low levels of immunoglobulin (Ig) E.
However, long-term macrolide treatment clearly has major disadvantages, including elevated bacterial resistance. Macrolide resistance rates were reported as 9.8 per cent in France and 13.2 per cent in Japan.Reference Peuchant, Ménard, Renaudin, Morozumi, Ubukata and Bébéar7, Reference Morozumi, Iwata, Hasegawa, Chiba, Takayanagi and Matsubara8 Alarmingly, two studies from China recently found that 18.0 per cent of Mycoplasma pneumoniae isolates from children with respiratory tract infections were resistant to macrolides.Reference Xin, Mi, Han, Qin, Li and Wei9, Reference Liu, Ye, Zhang, Xu, Li and Zhu10 In addition, it is likely that long-term, low-dose macrolide treatment is insufficient for patients with chronic rhinosinusitis of the non-neutrophilic type, who have a high risk of atopy (high levels of IgE, and/or high eosinophil counts in peripheral blood and nasal mucosa). For example, Videler et al. reported no significant benefit over a placebo in 60 chronic rhinosinusitis patients in a recent study focusing on the efficacy of low-dose azithromycin.Reference Videler, Badia, Harvey, Gane, Georgalas and van der Meulen11 The possible reasons for this (as suggested by the authors of that study) included the degree of disease severity in the investigated group, an under-dosage of azithromycin and ‘under-powering’ of the study.Reference Videler, Badia, Harvey, Gane, Georgalas and van der Meulen11 It remains unclear whether the observed lack of efficacy for patients with chronic rhinosinusitis of the non-neutrophilic type was because of an under-dosage of macrolides.
The current study aimed to establish whether short-term, high-dose clarithromycin was a more effective macrolide treatment protocol for chronic rhinosinusitis in patients with atopy than low-dose clarithromycin. A randomised, comparable investigation was conducted. We specifically wanted to examine the short-term clinical efficacy of high-dose clarithromycin for the treatment of patients with chronic rhinosinusitis of the non-neutrophilic type. Accordingly, we examined the effect of clarithromycin treatment on T-helper type 2 cytokine IL-5 in nasal secretion to evaluate its immunomodulatory property. Our findings might be beneficial in guiding the clinical use of macrolides as a treatment for chronic rhinosinusitis.
Materials and methods
Patients
This study was approved by the local ethics committee, and informed consent was obtained from each patient.
A total of 43 chronic rhinosinusitis patients without nasal polyps were enrolled in this study. All patients were required to be at least 20 years of age (of either sex). They had at least a 12-week history of 2 or more symptoms that included either nasal obstruction or nasal secretion. Other symptoms included: postnasal drip, facial pain or pressure, headache, and the reduction or loss of sense of smell. All patients had visible secretion in the middle nasal meatus and/or olfactory cleft (found by endoscopic examination), without polyps. Patients were required to be in good health, and free of any clinically significant disease that would interfere with the study schedule or procedures, or compromise the patients' safety. Atopy was confirmed via a positive skin prick test response (of class 2 or higher) to house dust mites or other common allergens, or positive serum IgE-mediated allergic reactivity (of class 2 or higher) specific to house dust mites or other common allergens. In order to be included in the study, patients must have been willing to give informed consent, and to adhere to visiting schedules and medication restrictions.
The exclusion criteria included the following: (1) cystic fibrosis, congenital ciliary dyskinesia, sinonasal fungal disease, systemic vasculitis, granulomatous disease, tumour, immunodeficiency or nasal polyps; (2) an allergy to clarithromycin; (3) a recent history of upper respiratory tract infection (within the four weeks prior to the study); (4) a serious metabolic, cardiovascular, autoimmune, neurological, blood, digestive, cerebrovascular or respiratory system disease; (5) any other disease that might interfere with the evaluation of the results or affect patient safety, such as glaucoma and tuberculosis; (6) mental disorders; (7) a recent history of local or systemic medication for chronic rhinosinusitis, such as glucocorticoids, macrolides and nasal irrigation (within the four weeks prior to the study); (8) a history of nasal sinus surgery; (9) a recent history of an acute asthma attack requiring admission to a hospital (within the four weeks prior to the study).
Study design
Demographic data, case history, and information regarding current and previous treatments for chronic rhinosinusitis were collected. The patients were then instructed to discontinue any treatment specific to chronic rhinosinusitis for two weeks (medication and/or additive specific measures taken at home).
All patients were reviewed on day 14 (baseline visit). Diary cards from the run-in period were collected and checked carefully. The patients were subsequently randomised to one of two groups to receive either low-dose clarithromycin (250 mg, once daily for 14 days) (Klacid; Abbott China, Shanghai, China) or high-dose clarithromycin (500 mg, twice daily for 7 days, followed by 250 mg twice daily for 7 days). The first medication tablet was administered on the day of the baseline visit.
Diary cards and quality of life (QoL) questionnaires were given to all patients to be completed over the four weeks that followed. These were collected and reviewed after 14 days of treatment (week 2 visit) and 14 days after the end of treatment (week 4 visit). The primary efficacy outcome measures were: individual nasal symptom scores (for nasal congestion, rhinorrhoea, postnasal drip and loss of smell) and total nasal symptom scores. The secondary efficacy outcome measures were: endoscopic evaluation (Lund–Kennedy scores), a QoL questionnaire (the Sino-Nasal Outcome Test 20 scores) and cytokine levels (IL-5 and IL-8) in nasal secretions.
Outcome measures
Symptom assessment
In this study, individual nasal symptom scores (nasal congestion, rhinorrhoea, postnasal drip and loss of smell) and total nasal symptom scores were recorded on a 0–10 cm visual analogue scale, wherein 0 cm represented ‘no complaints whatsoever’ and 10 cm represented ‘the worst imaginable complaints’. Clinical efficacy was determined based on the nasal symptom scores recorded by the patient at baseline (week 0), at the end of treatment (week 2 visit) and 14 days after the end of treatment (week 4 visit).
Endoscopic evaluation
Endoscopic inspection was performed by the senior investigator. The presence or absence of discharge and swelling, particularly in the middle meatus and olfactory cleft, was noted. Endoscopic scores for nasal discharge and mucosa were recorded according to the Lund–Kennedy system. (For nasal discharge: 0 = no discharge; 1 = clear, thin discharge; and 2 = thick or purulent discharge. For nasal mucosa: 0 = no swelling, 1 = mild swelling and 2 = severe swelling.) The total Lund–Kennedy scores for nasal discharge and nasal mucosa from the two sides were added together.
Quality of life questionnaire
Patients completed the Sino-Nasal Outcome Test 20 questionnaire at weeks 0, 2 and 4. As we have previously described, the Sino-Nasal Outcome Test 20 is a validated, rhinosinusitis-specific QoL instrument, in which patients are asked to answer 20 questions regarding their nasal symptoms and QoL.Reference Luo, Chen, Liu, Li, Xu and Fan6
Cytokine levels
Nasal secretions were collected (in the manner previously describedReference Luo, Chen, Liu, Li, Xu and Fan6) at the three time points. Concentrations of IL-5 and IL-8 were quantified using a FlowCytomix kit (eBioscience, San Diego, California, USA). The detection limits for IL-5 and IL-8 were 1.6 pg/ml and 0.5 pg/ml, respectively.
Statistical analysis
All data are expressed as medians and interquartile ranges. Data were analysed using the Kruskal–Wallis H test and the non-parametric Mann–Whitney U test. A p value of less than 0.025 (after Bonferroni correction) was defined as statistically significant, in order to avoid type I errors.
Results
Epidemiology
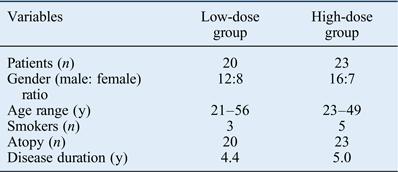
A total of 43 chronic rhinosinusitis patients were enrolled in this study (28 males and 15 females, aged between 21 and 56 years (mean of 31.7 years)). The patient demographics are shown in Table I. No significant differences were observed between the high- and low-dose clarithromycin groups for age, gender, smoking status or asthma co-morbidity.
Primary efficacy outcome measures
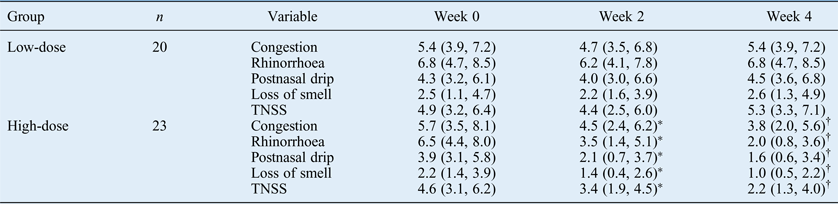
With regard to the low-dose group, no significant differences were observed between individual or total nasal symptom scores at weeks two or four and the baseline values (Table II). However, in the high-dose group, individual and total nasal symptom scores were significantly decreased at weeks two and four compared with the baseline values. Furthermore, compared with the values at week two, individual and total nasal symptom scores were significantly decreased at week four in the high-dose group. Significant differences in individual and total nasal symptom scores were also observed between the low-dose and high-dose groups at week two and week four.
Table II Nasal symptom scores

Data values represent median visual analogue scale scores and interquartile ranges. *p < 0.025, compared with baseline values. †p < 0.025, compared with values at week two. TNSS = total nasal symptom score
Secondary efficacy outcome measures
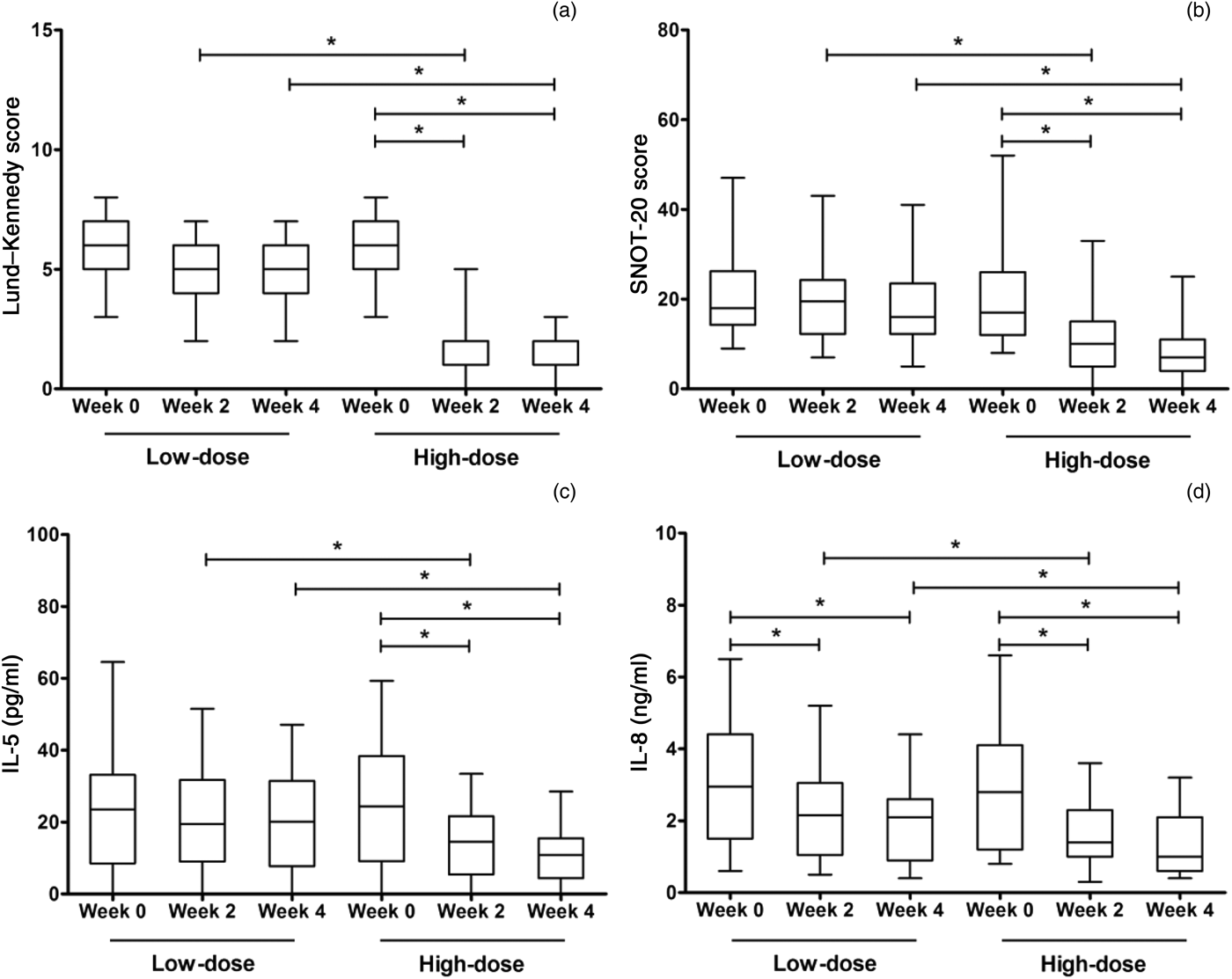
With regard to the low-dose group, no significant differences were observed between the Lund–Kennedy scores or Sino-Nasal Outcome Test 20 scores at weeks two or four and the baseline values (Figure 1). However, in the high-dose group, these scores were significantly decreased at weeks two and four compared with the baseline values. Significant differences were observed between the low-dose and high-dose groups for the Lund–Kennedy scores and the Sino-Nasal Outcome Test 20 scores at week two and week four.

Fig. 1 Results for secondary efficacy outcome measures for the high- and low-dose clarithromycin treatment groups at weeks 0, 2 and 4, showing changes in (a) Lund–Kennedy scores, (b) Sino-Nasal Outcome Test 20 scores, (c) IL (interleukin)-5 concentration and (d) IL-8 concentration. Data values represent median scores and interquartile ranges. *p < 0.025. SNOT-20 = Sino-Nasal Outcome Test 20; IL = interleukin
The IL-8 levels in nasal secretions were significantly decreased at weeks two and four compared with the baseline values, for both the low- and high-dose groups. Furthermore, in the high-dose group (but not the low-dose group), IL-5 levels were significantly decreased at weeks two and four compared with the baseline values. In addition, significant differences were observed between the low-dose and high-dose groups for the IL-5 and IL-8 levels at week two and at week four.
Discussion
Although long-term, low-dose macrolide treatment has been suggested for the treatment of chronic rhinosinusitis and other respiratory diseases, the lack of evidence and the adverse effects (such as increased macrolide resistance) should not be ignored. In the present study, we found that low-dose clarithromycin administration was ineffective for chronic rhinosinusitis patients after two weeks of treatment. However, the short-term, high-dose protocol (500 mg, twice daily for 7 days, followed by 250 mg, twice daily for 7 days) was found to result in clinical improvement. High-dose clarithromycin administration was associated with significant decreases in individual and total nasal symptom scores, Lund–Kennedy scores, Sino-Nasal Outcome Test 20 scores, and levels of IL-5 and IL-8 in nasal secretions at weeks two and four compared with the baseline values. Our findings thus demonstrate for the first time that a short-term, high-dose clarithromycin protocol is an effective alternative chronic rhinosinusitis treatment.
Since the introduction of long-term, low-dose macrolide protocols more than 20 years ago, reports have demonstrated the clinical efficacy of macrolide administration for treating chronic rhinosinusitis that is incurable by surgery or glucocorticoid treatment.Reference Cervin, Wallwork, Mackay-Sim, Coman and Greiff5, Reference Luo, Chen, Liu, Li, Xu and Fan6, Reference Videler, Badia, Harvey, Gane, Georgalas and van der Meulen11–Reference Suzuki, Ikeda, Honma, Gotoh, Oshima and Furukawa13 Macrolide therapy has been shown to have a slow onset, with ongoing improvement until several months after the start of therapy.Reference Cervin and Wallwork3 However, we have observed significant clinical improvement in chronic rhinosinusitis patients after clarithromycin treatment over two to four weeks, both in this study and in our previous study.Reference Luo, Chen, Liu, Li, Xu and Fan6 This suggests that a short-term, high-dose protocol of clarithromycin treatment may also be an effective option. In the high-dose group, we found that nasal symptom scores, Lund–Kennedy scores, Sino-Nasal Outcome Test 20 scores, and levels of IL-5 and IL-8, were significantly decreased at week two compared with the baseline values. This indicates that it is the dosage and not the length of treatment that is essential for effective clarithromycin treatment. Moreover, as individual and total nasal symptom scores differed significantly between the low-dose and high-dose groups, this suggests that the high-dose protocol carries a significant advantage over the low-dose protocol.
The molecular mechanisms underlying macrolide therapy are not completely understood. In animal studies, macrolides have increased mucociliary transport, reduced goblet cell secretion, inhibited IL-6 and IL-8 gene expression, and accelerated neutrophil apoptosis.Reference Tamaoki, Nakata, Tagaya and Konno14 There is also in vitro evidence showing that macrolides reduce the virulence and tissue damage caused by chronic bacterial colonisation, without eradicating the bacteria.Reference Cervin and Wallwork15 The majority of the uncontrolled investigations of macrolide therapy have suggested clinical benefits, improvements in symptoms and endoscopic findings, and reductions in neutrophil infiltration and IL-8 levels in nasal discharge.Reference Suzuki, Shimomura, Ikeda, Oshima and Takasaka16, Reference Yamada, Fujieda, Mori, Yamamoto and Saito17
Recent studies have demonstrated that macrolides are also effective for treating eosinophil-predominant allergic inflammation, such as nasal polyps and asthma. For example, Hrvacić et al. reported that clarithromycin treatment (200 mg/kg administered intraperitoneally) decreased IL-4, IL-5 and IL-13 concentrations in bronchoalveolar lavage fluid, suggesting that clarithromycin pre-treatment can modulate the T-helper type 2 response and eosinophilic inflammation.Reference Hrvacić, Bosnjak, Bosnar, Ferencić, Glojnarić and Eraković Haber18 In addition, Lin et al. reported that azithromycin can preferentially down-regulate IL-5 production in vitro, indicating its therapeutic potential for controlling childhood asthma.Reference Lin, Lee, Liang, Yan, Cheng and Kuo19 More interestingly, Park et al. recently reported that macrolides effectively inhibited myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts.Reference Park, Park, Cho, Lee and Lee20
In this study, we demonstrated for the first time that high-dose clarithromycin treatment (over two to four weeks) significantly inhibited both IL-8 and IL-5 concentrations in nasal secretions; this reduction in IL-5 levels was not found for the low-dose group. High-dose clarithromycin treatment was also associated with better clinical efficacy than the low-dose treatment. In an attempt to exclude the possible antibiotic effect of high-dose clarithromycin treatment, all patients in this study were confirmed to be atopic. Our findings thus demonstrate that the clinical efficacy of clarithromycin treatment for chronic rhinosinusitis patients was associated with its anti-inflammatory properties and a consequent inhibition of T-helper type 2-type inflammation. In agreement with our findings, Peric et al. recently reported that a higher-dose clarithromycin treatment (500 mg/day over eight weeks) decreased the size of nasal polyps in 45.45 per cent of non-atopic patients and in 50 per cent of atopic patients, demonstrating similar clinical effects in allergic and non-allergic patients.Reference Peric, Vojvodic, Baletic, Peric and Miljanovic21
• Low-dose, long-term clarithromycin has been recommended for treatment of chronic rhinosinusitis without nasal polyps
• This study suggests short-term, high-dose clarithromycin is a more effective protocol
• The anti-inflammatory properties of macrolides and consequent inhibition of T-helper type 2 inflammation are highlighted
We acknowledge that this study is not without flaws. First, no placebo group was included for the efficacy measures. Second, the clarithromycin dose prescribed was higher, and the duration of therapy and follow up was shorter than is usual in practice. Third, the antibiotic effect of macrolide cannot be excluded; further study is needed to address this possibility. Nonetheless, the results indicate that establishing a short-term, high-dose macrolide protocol for chronic rhinosinusitis patients might help to improve clinical efficacy and prevent macrolide resistance.
Conclusion
Our study indicated that short-term, high-dose clarithromycin was more effective than low-dose clarithromycin for the treatment of atopic chronic rhinosinusitis patients. These findings support a short-term, high-dose macrolide protocol for chronic rhinosinusitis patients.
Acknowledgements
This study was supported by a National Natural Science Grant of China (No. 81070771, 81070772, 81271054), and grants from the Ministry of Hygiene (No. 201202005) and Program for New Century Excellent Talents in University (No. NCET-10-0851).