1. Introduction
Methanesulfonic acid (CH3SO3H) is an important compound formed by the oxidation of dimethyl sulfide (DMS) in the atmosphere (Reference Hynes, Wine and SemmesHynes and others, 1986; Reference Saigne and LegrandSaigne and Legrand, 1987; Reference Saltelli and HjorthSaltelli and Hjorth, 1995). CH3SO3H is specific to marine biogenic activity, so its concentration in the remote troposphere has been a subject of great interest over the past decade. In ice cores, CH3SO3H can be detected through ion chromatography (CH3SO3 -) by melting an ice sample. Its anion is of primary importance as a tool for reconstructing several activities related to climate change: marine productivity (Reference Legrand and Feniet-SaigneLegrand and others, 1991, Reference Legrand, Feniet-Saigne, Saltzman, Germain, Barkov and Petrov1992), sea-ice extent (Reference Curran, van Ommen, Morgan, Phillips and PalmerCurran and others, 2003) and major El Nino events (Reference Legrand and Feniet-SaigneLegrand and Feniet-Saigne, 1991).
In the present time period, ice-core CH3SO3 - appears to derive mainly from post-depositional relocation, a process that occurs while the acid is in the gas and/or liquid state (Reference Mulvaney, Pasteur, Peel, Saltzman and WhungMulvaney and others, 1992;Reference Jaffrezo, Davidson, Legrand and DibbJaffrezo and others, 1994; Reference Pasteur and MulvaneyPasteur and Mulvaney, 1999, 2000; Reference McMorrow, van Ommen, Morgan and CurranMcMorrow and others, 2004; Reference Smith, van Ommen and CurranSmith and others, 2004; Ruth and others, 2005). On the other hand, a recent study using Raman spectroscopy has provided new information on the chemical forms of certain water-soluble salts contained in the microparticles of ice cores. This evidence suggests that methanesulfonate may exist as a salt in glacial period ice (Reference Ohno, Igarashi and HondohOhno and others, 2005, Reference Ohno, Igarashi and Hondoh2006; Reference Barletta, Gros and HerringBarletta and others, 2009). However, very little is known about the eutectic temperature and Raman spectra of methanesulfonate salts. This study identifies methanesulfonate salt particles in the Dome Fuji (Antarctica) ice core using micro-Raman spectroscopy, and proposes a formation process based on phase diagrams measured by differential scanning calorimetry (DSC) and Raman spectroscopy.
2. Methods
2.1. Chemical form of methanesulfonate salts
Our micro-Raman spectrometer is a triple monochromator (Jobin-Yvon, T64000) equipped with a charge-coupled device (CCD) detector (Jobin-Yvon, Spectraview-2D). The device has good quantum efficiency from 500 to 900 nm. The spectral resolution of the Raman spectrum is 0.6 cm-1. The absolute frequency of the monochromator was calibrated using a neon emission line and silicon wafers. The cross slit of this measurement is 3 mm. The cryostat is posed on an x-y-z translation stage, and fed by a N 2 gas flow from liquid nitrogen heated inside a dewar. More detgails of the method can be found in Reference GenceliGenceli and others (2009) and Sakurai and others (in press).
The microparticles in the Dome Fuji ice core were analyzed using micro-Raman spectroscopy. The Dome Fuji ice-core section at Termination I (the Holocene/Last Glacial Maximum (LGM) transition) was cut with a bandsaw at depths of 340.38 (Holocene), 382.86, 414.36, 463.85, 502.35 (Termination I) and 576.50 m. The final depth corresponds to the LGM. All sections have dimensions of 10 × 10 × 3 mm3. The Raman spectra of microparticles in the ice core were compared with Raman spectra taken from reference specimens of simple salts such as reagent-grade chemicals 98wt% CH3SO3Na (Alfa Aesar), 99wt% (CH3SO3)2Mg (Daniels Fine Chemicals), 98wt% CH3SO3K (Tokyo Kasei Kogyo) and 98 wt% (CH3SO3)2Ca (Tokyo Kasei Kogyo) at room temperature. CH3SO3Na-H2O, (CH3SO3)2Mg-H2O and CH3SO3K-H2O were measured by Raman spectroscopy at -30°C, and (CH3SO3)2Ca-H2O was measured at -50°C, temperatures below the eutectic temperature, at which solid hydrated crystal salts formed. All four solutions were prepared with ultrapure water (18.3 mfi) by weighing out the required amounts of the aforementioned reagent-grade chemicals. Due to their high percentage purities, we assume that these chemicals are anhydrous. Note that we distinguish the spectra on the basis of peak shapes as well as peak positions.
2.2. The eutectic temperatures of methanesulfonate salts
The phase diagrams of the methanesulfonate salts, including their eutectic temperatures, are determined using a differential scanning calorimeter (DSC;Rigaku Co., DSC8230) with liquid nitrogen cooling. Further details on the analytical technique of DSC measurement are provided by, for example, Reference WunderlichWunderlich (1973). The temperature reproducibility of this instrument is better than ±0.2°C, based on a three-point temperature calibration using indium (156.6°C), water (0.0°C) and cyclohexane (-83.0°C) (Reference CharsleyCharsley, 1993).
The DSC measurements are carried out on specimens of CH3SO3Na-H2O, (CH3SO3 Mg-H2O, CH3SO3K-H2O and (CH3SO3)2Ca-H2O. Solutions are prepared by combining ultrapure water (18.3 mO) with reagents. The DSC cells are made of aluminum and have a capacity of 30pL. In cases where the samples sublimated, the cells were sealed before use. During the measurements for CH3SO3Na-H2O, (CH3SO3)2Mg-H2O and CH3SO3K-H2O, the cells were cooled to -70°C at a rate of 20°Cmin-1 and then heated to room temperature at 10°Cmin-1. These three types of samples typically had a mass of about 2 mg. The (CH3SO3)2Ca-H2O samples typically had a mass of about 15 mg. The (CH3SO3)2Ca-H2O cells were cooled to-80°C at a rate of 20°C min-1 and then heated to room temperature at a rate of 1°Cmin-1. These conditions were chosen because the latent heat of (CH3SO3)2Ca-H2O is very low.
3. Results
3.1. Raman spectra of methanesulfonate salts
We measured the Raman spectra of several reference specimens: the simple salts CH3SO3Na, (CH3SO3)2Mg, CH3SO3K and (CH3SO3)2Ca (Fig. 1), and their solid hydrated crystal salts with ice under eutectic conditions (Fig. 2). The methanesulfonate salts show three major peaks around 1050-1100, 2930-2950 and 3010-3030 cm-1. The peak around 1050-1100 cm-1 can be assigned to the SO3 2- stretching frequencies (Reference SocratesSocrates, 2001; Reference Barletta, Gros and HerringBarletta and others, 2009). The peaks around 2930-2950 and 3010-3030 cm-1 can be assigned to CH3 symmetric stretching and CH3 asymmetric stretching respectively (Reference Durig, Zhou, Schwartz and GounevDurig and others, 2000; Reference SocratesSocrates, 2001;Reference Barletta, Gros and HerringBarletta and others, 2009).
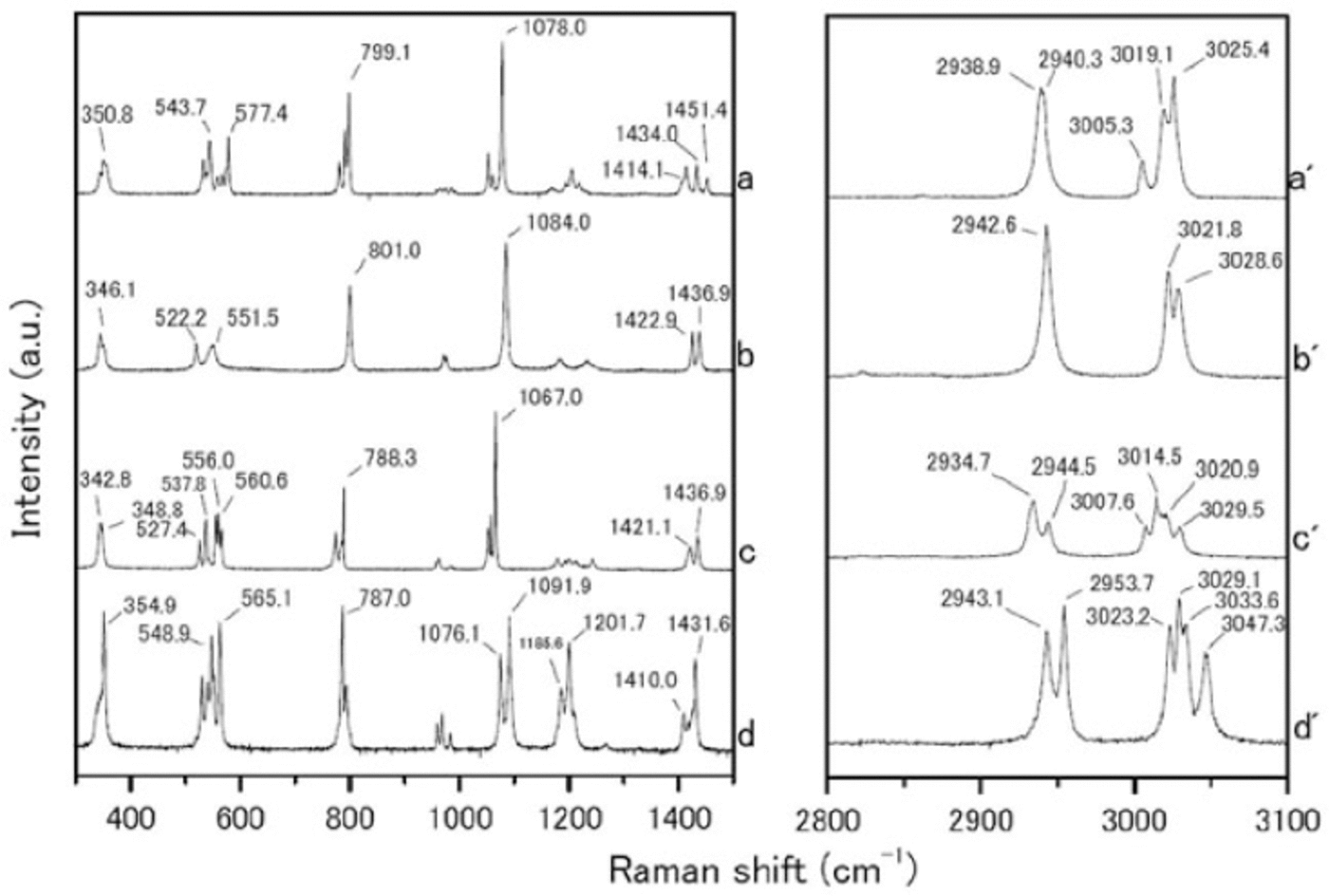
Fig. 1. Raman spectra from (a-d) 300-1500 cm-1 and (a’-d’) 2800-3100 cm-1. (a-d) are reagent-grade crystals of CH3SO3Na, (CH3SO3)2 Mg, CH3SO3K and (CH3SO3)2 Ca respectively, which were measured at room temperature.
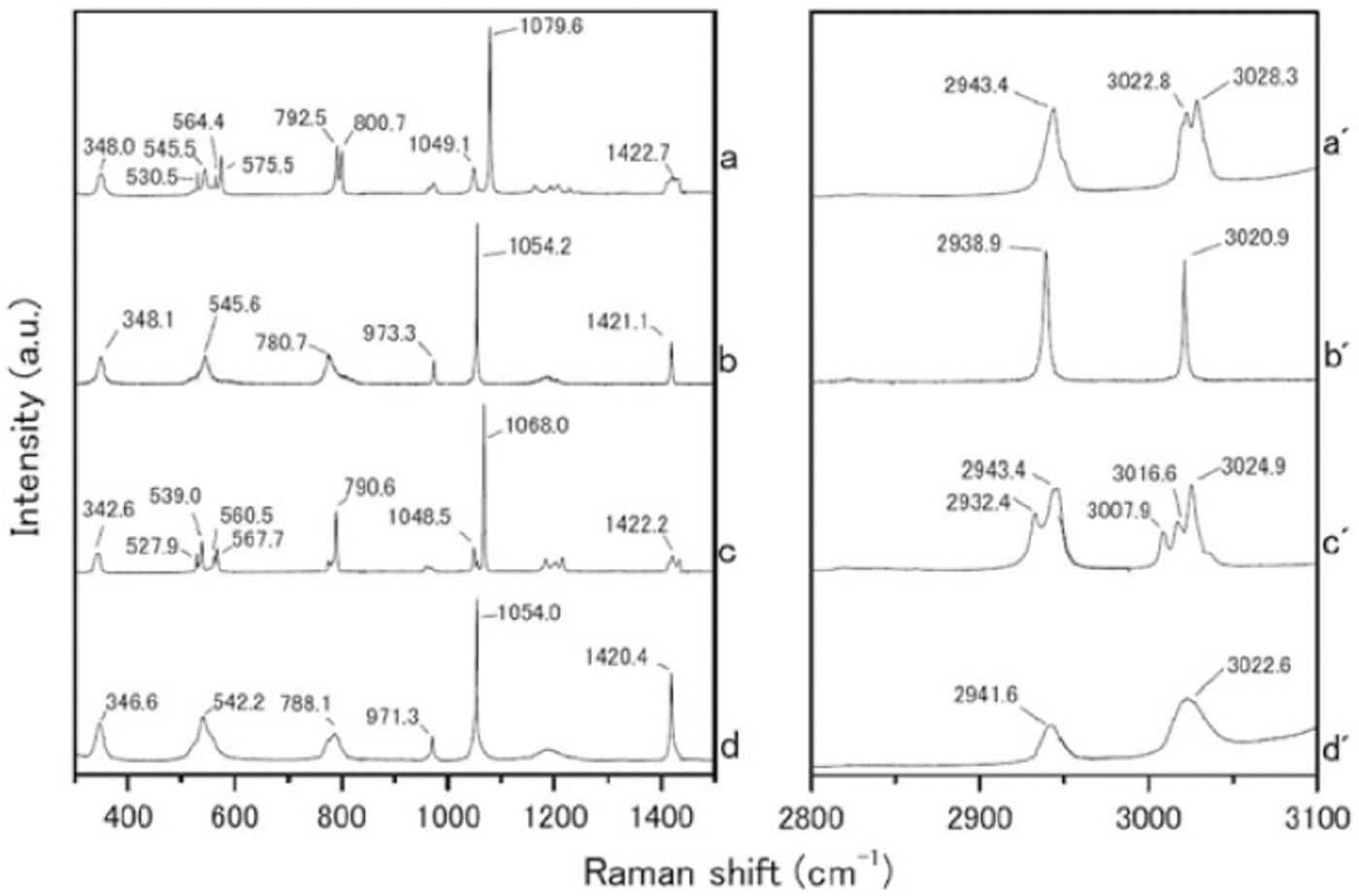
Fig. 2. Raman spectra from (a-d) 300-1500 cm-1 and (a’-d’) 2800-3100 cm-1. (a-d) are hydrated crystals of CH3SO3Na nH2O, (CH3SO3)2Mg nH2O, CH3SO3K nH2O and (CH3SO3)2 Ca nH2O respectively, which formed solid hydrated crystal salt under eutectic conditions. Measurement temperatures were -30_C for CH3SO3 Na nH2O, (CH3SO3)2 Mg nH2O and CH3SO3K nH2O, and -50_C for (CH3SO3)2 Ca nH2O.
The Raman spectra of the simple salts are significantly different from those of the corresponding hydrated crystals. The differences are especially evident in the SO3 2-, OH and CH3 stretching frequencies. The SO3 2- stretching mode generates a peak at 1084.0 cm-1 for the (CH3SO3)2Mg salt. The (CH3SO3)2Mg-H2O solution, on the other hand, has a peak at 1054.2 cm-1. The OH stretching mode does not appear for any simple salt. In contrast, all the solutions have a large peak around 3000-3500cm-1 from OH stretching modes. A more interesting result from these measurements relates to the CH3 symmetric and asymmetric stretching modes from 3010 to 3030cm-1. In Raman spectra from simple salts, we detect mode splitting. For instance, the spectrum of (CH3SO3)2Mg has peaks at 3021.8 and 3028.6cm-1 from CH3 asymmetric stretching (Fig. 3a). The (CH3SO3)2Mg-H2O sample, on the other hand, produces a single sharp peak at 3020.9 cm-1. These disagreements must be caused by the crystal structure, suggesting that hydrate is forming in the methanesulfonate salt, i.e. the structure in solution is (CH3SO3)2Mg-nH2O.

Fig. 3. Optical microcopy of a methanesulfonate salt particle (arrow) found in the ice sample at 576.5 m depth (24.5 ka BP). The scale bar is 10 μm. The Raman spectra from (a-d) 200-1500 cm-1 and (a’-d’) 2500-3100 cm-1 shown below the photograph come from (a) (CH3SO3)2 Mg, (b) (CH3SO3)2 Mg nH2O (after standing at room temperature for several days and measures at -30_C), (c) (CH3SO3)2 Mg nH2O and (d) the methanesulfonate salt inclusion from the Dome Fuji ice core (the particle in the photograph).
3.2. Chemical form of methanesulfonate salts in the ice core
We found microparticles with Raman spectra very similar to those of the methanesulfonate salt (CH3SO3)2Mg. Among the six depths considered, particles of this kind are only present in LGM (576.50 m depth) ice. Furthermore, no other types of methanesulfonate salt were found among the 758 microparticles examined (Fig. 4). The microparticles of (CH3SO3)2Mg were typically several microns in diameter (Fig. 3 photograph).

Fig. 4. The distribution of microparticle types found in ice samples taken from Termination I of the Dome Fuji ice core. The climate ranges are 340 m (Holocene), 382-502 m (Termination I) and 576 m (LGM). The numbers of microparticles measured at each depth are 87, 106, 101, 161, 237 and 66 (a total of 758 particles.) The numbers of microparticles in the ‘gypsum’ category are 6, 11, 11, 73, 69 and 27 respectively. The numbers in the ‘mirabilite and meridianiite’ category are 58, 90, 86, 29, 77 and 1 respectively. Only four (CH3SO3)2Mg nH2O microparticles were found, and these only in the LGM.
To identify the chemical form of the methanesulfonate salts present in the ice core, we compared the Raman spectra of microparticles (Fig. 3) with the reference spectra. The Raman spectra of the methanesulfonate salt particles found in the Dome Fuji ice correspond with the (CH3SO3)2Mg-nH2O reference specimen, not with the anhydrous (CH3SO3)2Mg specimen or any other chemical form.
The Raman spectra of (CH3SO3)2Ca-nH2O and (CH3SO3)2Mg-nH2O are very similar in many respects. However, they can be distinguished by their symmetric and asymmetric CH3 stretching frequencies: 2938.9 and 3020.9 cm-1 for (CH3SO3)2Mg-nH2O, or 2941.6 and 3022.6cm-1 for (CH3SO3)2Ca-nH2O. We used Gaussian fitting to estimate the positions and half-bandwidths of the peaks. The corresponding peaks in Raman spectra obtained from methanesulfonate salt particles in the ice core are located at 2938.9 and 3020.9 cm-1. The half- bandwidths of the peaks are 3.9 ± 0.1 and 2.9 ± 0.9cm-1 respectively. The peak locations and half-bandwidths of the (CH3SO3)2-Ca-nH2O reference hydrated crystal are 2941.6cm-1, 3023.1 cm-1 and 15.8 ± 0.2cm-1, 23.7 ± 3.4 cm-1 respectively. This evidence strongly suggests that the methanesulfonate salt found in the ice core is (CH3SO3)2Mg-nH2O and not (CH3SO3)2Ca-nH2O.
To evaluate the structural phase transition during preservation, we left the (CH3SO3)2Mg-H2O solution in a Petri dish at room temperature for several days to sublimate the water, then measured the Raman spectrum of the sample. The resulting spectrum remains clearly (CH3SO3)2Mg-nH2O, not (CH3SO3)2Mg (Fig. 3b and c). This result suggests that once (CH3SO3)2Mg-nH2O has formed it can be preserved as a structure of hydrate. It further suggests that a structural phase transition to the anhydrous state or some other phase could not have occurred.
3.3. Phase diagrams of methanesulfonate salts
The phase transitions of CH3 SO3 Na-H2 O, (CH3SO3)2Mg-H2O, CH3SO3K-H2O and (CH3SO3)2Ca-H2O generate typical DSC profiles during the heating process. The phase diagrams of the solutions are shown in Figure 5.
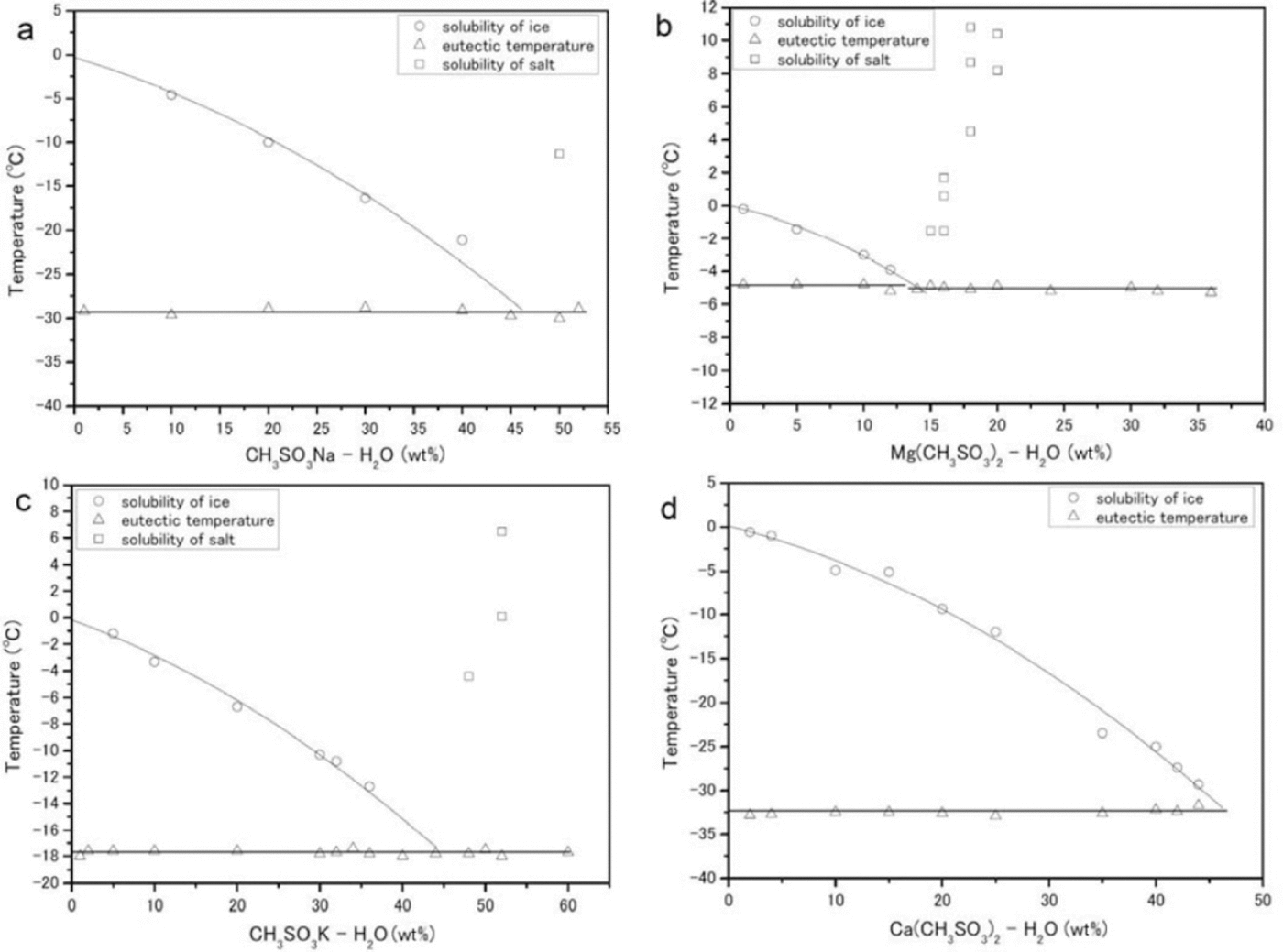
Fig. 5. Phase diagrams (eutectic temperatures are given in parentheses) constructed from DSC measurements. (a-d) correspond to CH3SO3Na-H2O (-29.3 ± 0.2_C), (CH3SO3)2 Mg-H2O (-5.0 ± 0.5_C), CH3SO3K-H2O (-17.8 ± 0.1_C) and (CH3SO3)2 Ca-H2O (-32.6 ± 0.2_C) respectively. The circles, triangles and squares trace the solubility of ice, the eutectic temperature and the solubility of salt respectively.
CH3SO3Na-H2O
We performed measurements in the composition range 0-52 wt% (Fig. 5a). The eutectic temperature of this salt is -29.3 ± 0.2°C. Concentrations higher than 50wt% CH3SO3Na-H2O were deposited from the solution at room temperature. The enthalpy of fusion at the eutectic composition of 50wt% is ΔH Na = 12.6 ± 0.9 kJ mol-1.
CH3SO3)Mg-H2O
We performed measurements in the composition range 0-36 wt% (Fig. 5b). The average eutectic transition temperature over all samples is -5.0±0.5°C. Above a solute concentration of 14wt%, salt deposition occurred at room temperature. The enthalpy of fusion at the eutectic composition of 14 wt% is ΔH Mg = 13.3 ± 0.04 kJ mol-1.
CH3SO3K-H2O
We performed measurements in the composition range 0-60 wt% (Fig. 5c). The average eutectic transition temperature is -17.8 ± 0.1 °C. The enthalpy of fusion at the eutectic composition of 44wt% is ΔH K = 17.9± 0.08 kJ mol-1.
(CH3CO3)2 Ca-H2O
We performed measurements in the composition range 0-44 wt% (Fig. 5d). The average eutectic transition temperature is -32.6 ± 0.2°C. At compositions of 44wt% or higher, our measurements of this compound were not easily reproducible (although the other three species were detectable at their eutectic compositions). We therefore do not have a good estimate for the enthalpy of fusion, but one profile gave ΔH Ca = 0.46± 0.04 kJ mol-1.
4. Discussion
The eutectic temperature of (CH3SO3)2Mg-nH2O (-5.0± 0.5°C) is much higher than any temperature within the Dome Fuji ice core, from the surface to the lowest depth. The average temperature of the core at 10 m depth is -58°C, and the deepest recorded temperature (from the first Dome Fuji ice-core project, at 2503 m) is ˜-10°C (Reference Hondoh, Shoji, Watanabe, Salamatin and LipenkovHondoh and others, 2002). This result indicates that (CH3SO3)2Mg-nH2O is thermodynamically stable in the Dome Fuji ice core between the surface and 2503 m. We shall now propose two formation mechanisms for (CH3SO3)Mg-nH2O in Antarctica, one in the ice-sheet firn and the other in the polar atmosphere.
CH3SO3H is formed by the oxidation of DMS, which is emitted by biological activity (e.g. Reference Saltelli and HjorthSaltelli and Hjorth, 1995) on the open sea. Sea salts are emitted by sea spray and probably from surface sea ice (e.g. Reference Rankin, Wolff and MartinRankin and others, 2002). The chemical reaction of CH3SO3H with sea salt can form methanesulfonate salt. In previous studies using ion chromatography on coastal ice cores obtained at Dolleman Island and Siple Dome, Antarctica, (Reference Mulvaney, Pasteur, Peel, Saltzman and WhungMulvaney and others, 1992; Reference Kreutz, Mayewski, Whitlow and TwicklerKreutz and others, 1998) the concentration of CH3SO3 - in the winter layer (which was relocated from the summer layer) determined whether sodium methanesulfonate or magnesium methanesulfonate formed at a depth of several meters. The temperatures of the cores at 10m depth were -16.8°C and -25°C at Dolleman Island and Siple Dome respectively, suggesting that deeper ice might be at even higher temperatures. Taking into consideration our data on the eutectic temperatures of methanesulfonate salts, CH3SO3Na-nH2O (-29.3°C) could not be present in the Dolleman Island ice core but (CH3SO3)2Mg-nH2O (-5.0°C) should be.
The annual surface temperature (e.g. 1996/97) on the Dome Fuji ice sheet ranges from -79.7°C to -18.6°C, with a mean of -54.4°C (Reference Yamanouchi, Hirasawa, Hayashi, Takahashi and KanetoYamanouchi and others, 2003). The annual average surface temperature during the LGM has been determined from stable-isotope studies as roughly -60°C (Reference Watanabe, Jouzel, Johnsen, Parrenin, Shoji and YoshidaWatanabe and others, 2003). There should also have been a seasonal variation around this mean. From previous studies, Na+ is the most abundant ion, while Mg2+ and CH3SO3 - are minor ions in both periods, i.e. during the LGM and in current times (e.g. Reference Watanabe, Jouzel, Johnsen, Parrenin, Shoji and YoshidaWatanabe and others, 2003; Reference IizukaIizuka and others, 2004, Reference Iizuka, Hondoh and Fujii2006). Thus, these previous studies suggest that CH3SO3Na-nH2O can form at the surface of the Dome Fuji ice during both periods, and that (CH3SO3)2Mg-nH2O is probably a minor compound in the Dome Fuji ice core. However, among the 758 microparticles analyzed, we found no methanesulfonate salts except for (CH3SO3)2Mg-nH2O in LGM ice. The discrepancy can be explained by the surface temperature and acidity of the ice at the time it formed.
Taking into consideration the eutectic temperatures of the Mg and Na salts, LGM summer temperatures at the surface could have been high enough to melt CH3SO3Na-nH2O but not (CH3SO3)2Mg-nH2O. Furthermore, CH3SO3H might sublimate from the firn after the CH3SO3Na-nH2O melts, lowering the concentration of CH3SO3 - in the ice. This situation, dominant in the Holocene and in Termination I, suggests that (CH3SO3)2Mg-nH2O could have formed in the Holocene and Termination I sections of the Dome Fuji ice core. According to previous studies of the firn, however, sulfuric acid and sea salt can combine to produce sulfate salts (Reference Iizuka, Hondoh and Fujiilizuka and others, 2006). The high acidity of Holocene and Termination I ice (Reference Fujita, Maeno, Furukawa and MatsuokaFujita and others, 2002) may have allowed the reaction
to occur during the post-depositional process, eliminating (CH3SO3)2Mg-nH2O particles from the ice.
In the LGM ice, methanesulfonate salts form by fixation on alkaline particles of marine or continental origin during long- range aerosol transport to polar areas (Reference Delmas, Wagnon, Goto-Azuma, Kamiyama and WatanabeDelmas and others, 2003). During this period, high levels of dust neutralized the acids, greatly reducing their abundance (Reference RöthlisbergerRöthlisberger and others, 2003;Reference IizukaIizuka and others, 2008). This neutralization of the LGM ice permits the existence of (CH3SO3)2Mg-nH2O.
To summarize, we propose that (CH3SO3)2Mg-nH2O may have formed in the air and been transported as an aerosol. The salt was then deposited on the ice sheet, where it had a chance to survive and be preserved in LGM ice because the acids were neutralized.
5. Conclusion
We found (CH3SO3)2Mg-nH2O in the LGM ice from Dome Fuji, and this salt is restricted to the coldest period in the glacial cycle. Our study suggests that (CH3SO3)2Mg-nH2O formed in the atmosphere of Antarctica. In acidic Holocene ice, however, (CH3SO3)2Mg-nH2O was dissolved by acid after being deposited. Only during the LGM did (CH3SO3)2Mg-nH2O that formed in the atmosphere have a chance to survive on the ice sheet and be preserved in the Dome Fuji ice core.
Acknowledgements
We thank all participants in the Dome Fuji ice-core drilling project. We also thank A. Hori and A. Miyamoto for valuable discussions, and the scientific editor B. Kulessa. This study was supported by Grants-in-Aid for Creative Scientific Research (grant Nos. 14GS0202 and 21710002), by the Global COE program (Establishment of Center for Integrated Field Environmental Science) and by a Special Education Study Expense fund (for cooperation between universities) provided by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS), Japan.









