Introduction
The base of an alpIne glacier is sometimes plastered with a frozen mud layer. Such a mud layer has been observed at the ceiling of an enclosed natural subglacial cavity beneath the Glacier d’Argentière in the Western Alps (Fig. 1). Its thickness ranges from 2 to 3 cm. A large pressure gradient exists across this mud layer: interstitial water in the glacier at its upper surface is under the hydrostatic pressure of about 90 m of ice and, at its lower surface, under the atmospheric pressure of the cavity. This interstitial water is thus forced through the mud layer under a pressure gradient of about 3 bar cm−1 and refreezes in the form of ice accretions hanging at the ceiling (Souchez and others, 1973). This refreezing is the con sequence of the pressure dependence of the freezing point.
Sample description and treatment
With the aid of a recorder-equipped atomic absorption spectrophotometer (Perkin Elmer 303) chemical analyses for Na, K, Ca, and Mg were performed on:

Fig. 1. The ceiling of the subglacial cavity Studied showing the plastered mud layer. Its frozen state is apparent thanks to a detached fragment visible in the centre of the picture. The glacier bedrock contact is at the lower part.
-
(1) samples of water in contact with the mud layer corresponding to the situation before the forcing, and
-
(2) samples of ice accretions and water held at their surface corresponding to the situation after the forcing.
Many precautions were taken in order to reduce the contribution from contamination to the background noise level of the instrument, and to control chemical changes caused by the melting of the samples. Sampling procedure and sample treatment have been described previously in this Journal (Souchez and others, 1973).
Furthermore, determination of the adsorbed cations held on the surface of the fine particles (diameter inferior to 50 μm) of the mud layer has been done by the classical ammonium acetate method (Reference JacksonJackson, 1958, p. 84). We assume that the cations exchanged by this method are those present in the diffuse layer existing between the negatively charged mineral surfaces and the external solution. In this diffuse layer, cations are much more numerous than anions because of the negative charges of the mineral surfaces. Together with such a mineral surface, the diffuse layer forms an electrical double layer (also called a Gouy layer).
Result and comments
Table I gives the mean and extreme values of the concentrations in Na, K, Ca, and Mg of the samples. The ratios (Na+K)/(Ca + Mg), Na/K and Ca/Mg are also included.
A lower (Na+K)/(Ca + Mg), a lower Na/K, and a higher Ca/Mg ratio can be observed in the Gouy layer of the mud than in the water in contact corresponding to the situation before the forcing. This is to be explained by selective adsorption governed by the ionic charge of the cations and their hydrated radii. The order of replaceability of the major cations has been established as follows: Na+ < K + < Mg++ < Ca ++ (Reference CarrollCarroll, 1959). The variations of the above-defined ratios between the Gouy layers of the mud and the water in contact are consistent with this order. This supposedly indicates that an equilibrium has been reached before the passage through the mud layer.
Table I. Concentations of Na, K, Ca and Mg in the samoles collected at the base of the glacier
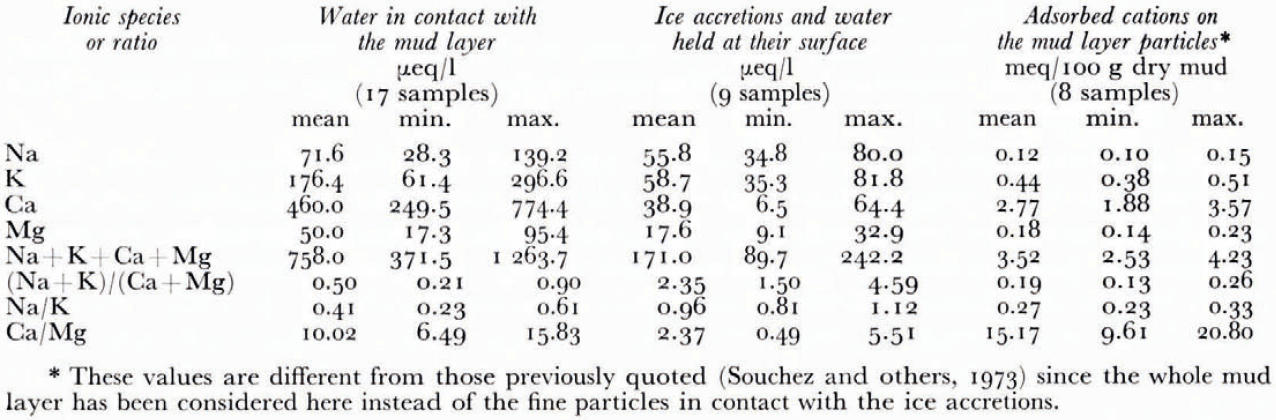
A comparison of the results between the samples of ice accretions and water held at their surface (a) and the samples of water in contact with the mud layer (b) leads to the following significant features:
-
a lower content in the four cations for (a) than for (b) showing a desalinating effect during the forcing;
-
a higher (Na + K)/(Ca + Mg) ratio for (a) than for (b) indicating a monovalent-divalent cation species separation;
-
a higher Na/K and a lower Ca/Mg ratio for (a) than for (b) indicating sorting effects between the alkalies as well as between the alkaline earths.
These characteristics show that the frozen mud layer plastered at the base of the glacier may be considered as an ion-exchanger membrane causing electrolyte nitration and relative retardation of cations (Reference HelfferichHelfferich, 1962).
These membrane properties are explained by the peculiar electrochemical situation in the pores of the mud layer. The water flows in the form of thin film s in which the ion concentration distribution is determined by the overlap of adjoining double layers. This distribution would also be approximately true in thin films of water adsorbed on single solid surfaces with air on the other side of the film (Reference KemperKemper, 1960). In these situations, the concentration of the anions becomes very low (Donnan exclusion). Forcing the water through the membrane builds up a counteracting streaming potential which accelerates the anions and slows down the cations. In the case of strong Donnan exclusion, electrolyte transfer across the membrane is weak, since the anions are scarcely admitted to the membrane in spite of the streaming potential and, because of the requirements of electroneutrality, the cations are more and more slackened. This electrolyte filtration effect is selective, i.e. the passage rates through the membrane are different for different cation species. Their relative passage rates will depend on the resultant of alt the forces affecting their selective adsorption and their selective trans port (Kharaka and Berry, 1973):
-
The forces of attraction between the cations and the electrical sites on the surface of the particles. In the electrolyte filtration process, increased strength of bonding of a cation will lead, everything else being equal, to its reduction in the effluent solution.
For example, divalent cations in the diffuse layer tend to be closer to the walls of the pores and, consequently, the average divalent cation is not carried down-stream as fast as the average monovalent cation (Reference Kemper, Kemper, Sills and AylmoreKemper and others, 1970).
-
The forces of repulsion between the cations and the streaming potential, which mainly depend on the ionic potential (charge/radius) of the hydrated cations. The cation with the higher ionic potential has to overcome the larger repulsive force and thus is the most retarded. As for the preceding factor, the relative retardation sequence is Na < K < Mg < Ca.
-
The dynamic force exerted on the cation by the flowing water, which is a direct function of its hydrated radius. The retardation sequence due to this factor is thus: Mg < Ca <Na < K.
Experimental work (Kemper and others, 1970) and field investigations (Reference BerryBerry, 1969; Reference Kharaka and BerryKharaka and Berry, 1973) indicate that with a low hydraulic pressure gradient, such as the one existing across the frozen mud layer at the base of the Glacier d’Argentière, the role of the dynamic force exerted by the flowing water can be neglected in comparison with the others, so that the relative retardation sequence to be considered is Na<K<Mg<Ca. This retardation sequence leads to a higher (Na+K)/(Ca + Mg) ratio, a higher Na/K ratio, and a lower Ca/Mg ratio for the effluent solution, which is the case at the base of the Glacier d’Argentière.
Now we must ask whether the calculated reduction of cationic content between the ice accretions and the water held at their surface and the water in contact with the mud layer is compatible with the model outlined above. The efficiency is of a membrane is defined as:

The efficiency of the mud layer for the four major cations considered as a whole is thus 77%. A lot of experimental work has been done to determine the efficiency of a clay membrane for solutions of various ionic strengths. However these experiments differ considerably from the situation described at the base of the Glacier d’Argentière because of the much higher pressure used. Nevertheless, Kemper (1960) uses a NaCl solution under 10 atmospheres, which is approximately the hydrostatic pressure at the base of the glacier, and gives the following values: E = 3% for a 1 N solution, E = 10% for a 0. 1 N solution and E = 32% for a 0.01 N solution. The same author indicates that the efficiency increases considerably with solutions of lower salinity. In the case studied here, the normality of the inflow solution for each cation considered is below 0.000 5 N. Thus an efficiency of 77% for such a dilute inflow solution is not in conflict with these experiments. Now, if we determine the membrane efficiency for each cation separately, we get the following figures: 23% for Na, 67% for K, 65% for Mg, and 92% for Ca. This is in agreement with the relative retardation sequence quoted above except for Mg; the efficiency for this element is here slightly lower than for K. However the significance of such a slight difference (2%) is questionable in view of the limited number of ice accretion samples which have been considered as valid after treatment (particle content of less than 0.5 g l−1).
The grain-size distribution of the mud layer indicates that it consists of sand with about 10% silt and 2% clay. The clay fraction is mainly illite as determined by X-ray diffraction. These characteristics are somewhat different from those of the electrolyte filtration experiments where a clay membrane is used. However, the mud layer is frozen at a temperature close to the melting point, so the large pores are filled with ice and unfrozen water exists partly at the interface between the mineral grains and ice and partly in crevices and capillaries where the radius of curvature is sufficiently small (Reference AndersonAnderson, 1967). In such a situation, the ions must migrate through thin films of unfrozen water which are continuous through the frozen mud (Reference Hoekstra and ChamberlainHoekstra and Chamberlain, 1964).
By comparing the situation before and after forcing across the mud layer, the chemical sorting effect postulated in Reference Souchcz, Souchez, Lorra and LemmensSouchcz and others (1973) is established. In comparison with this earlier paper, the reader will notice a difference in content of cations adsorbed on the particles in the mud layer. This is due to the fact that the whole mud layer has been sampled here instead of only the particles in contact with the ice accretions. So, the values reported in Table I of this paper are considered as more relevant.
Conclusion
A chemical sorting effect takes place at the base of the Glacier d’Argentière under the influence of a mud layer which acts as an ion-exchange membrane.
This effect is likely to occur in other glaciological situations. The basal part of an alpine glacier sometimes consists of stratified ice, i.e. interbedded layers of ice and frozen mud. Chemical analysis of this stratified ice may shed some light on its origin and so on the processes operating in the basal part of a glacier. The chemical composition of stratified ice is likely to be influenced by such a chemical sorting effect.






