Introduction
Participants are essential to any research study; without them, even the best-designed studies will not produce results. Attitudes and participation among the public, however, do not match this need. A survey by the Memorial Sloan Kettering Cancer Center indicated that only 35% of Americans said they were likely to enroll in a clinical trial while data from cancer-specific studies suggest that just 4% of cancer patients actually enroll in clinical trials annually [1]. Among clinical trial participants, disparities based on race/ethnicity are often observed with African-American and Hispanic cancer patients significantly less likely than White cancer patients to enroll in clinical trials [Reference Murthy, Krumholz and Gross2]. Research on clinical trial studies unrelated to cancer shows similar results [Reference Williams3, Reference Wilder4] suggesting wide-spread barriers to engaging patient populations into clinical trial research. If current levels of patient research participation continue, the goals and objectives of the National Institutes of Health (NIH) National Center for Advancing Translational Sciences regarding advancing clinical and translational research will go unrealized [Reference Kon5].
Many factors contribute to low research participation rates among adults in the general population including the rapid growth of available clinical trials [Reference Kolata6], restrictive eligibility criteria [Reference Garcia7], and that most patients get treatment with doctors and clinics who are not conducting clinical research [Reference Byrne8]. In addition to those barriers, minorities face additional challenges to participation. A meta-analysis of barriers to minority participation in research suggested mistrust, competing demands of time, unintended outcomes, lack of access to information, stigma, health insurance, and legal status concerns contributed to an unwillingness among racial/ethnic minorities to participate in clinical trials [Reference George, Duran and Norris9].The Tuskegee study, which exploited low-income African-American men for decades is a well-known source of mistrust [Reference Ford10]. Focus groups with Latinos suggest that language barriers and concerns about the privacy of documentation status are also significant impediments to their participation [Reference Ford10].
Several innovative approaches have been adopted with the goals of increasing clinical trial research enrollment and biomedical research more broadly [Reference Bryant11–Reference Cheah14]. Three of the most promising and cost-effective approaches being utilized in hospital and clinics settings include instituting universal or broad consent for research participation, on-site or web-based patient research registries and expanding the utility of patient portals to include research opportunities (see Table 1). Although differing in their approaches, each of these strategies is similar in that they seek to engage patients in hospital or clinical setting in research, to facilitate the identification and recruitment of large patient samples, and to establish large researchable databases comprised of clinical or biospecimen data.
Table 1 Three approaches to improving patient engagement in research
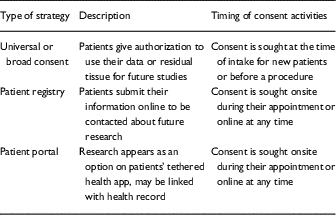
Universal consent for research participation represents the most wide-reaching of these approaches. With the recent release of the “Final Rule” for federally funded research, hospitals and other healthcare institutions are permitted to obtain universal consent to use patient demographic and clinical information or allow anonymized use of excess tissue and fluid specimens (biobank) for future research activities [Reference Menikoff, Kaneshiro and Pritchard15]. Universal consent for research is typically administered at the time of intake for new patients and/or before a procedure is performed. A form may ask patients to allow residual blood or tissue samples from procedures to be put into a biobank for research at a later time. Instead or in addition, the form may ask patients if they would like to be contacted to participate in future research studies. In both instances, information that can be used to determine study eligibility (such as demographics and diagnosis) may be kept, but it would be kept separately from any identifying information. Data from the Institute of Translational Health Sciences at the University of Washington showed that 65% of these patients asked to give universal consent for researchers to use their residual samples and 59% agreed to be contacted for future research studies [Reference Morneault16]. Another study from the Medical University of South Carolina indicated that 75% of patients asked agreed to residual specimen use and 72% agreed to be contacted for future research [Reference Marshall17]. For both types of consent, participation proportions were higher in Whites and males, though more females ended up in the database because more were contacted.
Patient registries are a second approach with potential benefit for increasing patient engagement in research. According to the Agency for Healthcare Research and Quality, “a patient registry is a data collection tool or database that contains information about patients’ medical conditions and/or treatments” [Reference Gliklich, Dreyer and Leavy18]. Patients provide basic demographic and medical information; these records are kept separately from the patients’ electronic medical record (EMR). There are several models for sign-up, including allowing patients to sign up on their own at home, on their own in the clinic at a kiosk, or with help from staff at a clinic appointment. Available data suggest that patient registries are cost-effective [Reference LeBlanc13, Reference Cheah14], increase patient involvement in research [Reference Callard12, Reference Cheah14] have high consent rates [Reference LeBlanc13, Reference Druce19], and improve patient autonomy [Reference Callard12]. For example, data from Rimel et al. suggest that online registries can boost participation by more than 4 times compared with paper registries, with higher participation from minorities [Reference Rimel20]. In a study of 5 clinics in Australia, 69.4% of all survey completers (43% of all patients approached) who were approached and asked to complete a tablet-based survey agreed to be contacted about future research [Reference Bryant11].
Patient portals “consist of provider-tethered applications that allow patients to electronically access health information that are documented and managed by healthcare institutions” [Reference Irizarry21]. In this approach, research is added as a separate tab on the interface, where patients review the information and agree to be contacted about future research for which they may qualify. A key difference from the patient registries is that patient portals are connected to the patient’s EMR which can be used to identify potentially eligible patients using more extensive clinical information (e.g., EMR verified stage of breast cancer diagnosis vs. a self-reported history of breast cancer). Among more than 11,000 users of MyChart at the University of Texas Southwestern Medical Center, 67% agreed to be contacted about future research studies, 24% said not at this time, and just 10% declined to participate at all [Reference Wilkinson22]. However, participation rates may not be equal: a 2013 meta-analysis found that patient portal use was lower among racial and ethnic minorities and those with lower education levels or literacy [Reference Goldzweig23].
To date, there are limited data and therefore no consensus regarding which, if any, of these potential approaches for increasing clinical research participation should be recommended or adopted in clinical settings. Further, the patient populations at those institutions that have implemented one or more of these strategies and published reports on their success may not reflect all academic medical centers. Community engagement has been identified as invaluable in enhancing participation in clinical trial research. The Clinical and Translational Science Award (CTSA) affiliated community based advisory boards are instrumental in advising best practices for engaging diverse patient populations. In order to make informed decisions about clinical based recruitment approaches, the Center for Clinical and Translational Science at the University of Illinois at Chicago (UIC) convened a long-standing community engagement advisory group to obtain feedback on the feasibility and acceptability of 3 approaches for increasing patient research enrollment—universal consent, patient registries, and patient portals. Secondary goals of the study were to discuss strategies for educating and engaging diverse patient groups with the recruitment approaches deemed as most feasible and acceptable to diverse population groups.
Materials and Methods
Participants
Funded by the National Institutes of Health, the Center for Clinical and Translational Science (CCTS) is the Clinical and Translational Science Award (CTSA) program at the UIC. CCTS seeks to accelerate the research process, enabling scientific discoveries to reach patient and populations faster. It also seeks to improve population health, particularly among minorities and underserved populations by, “Collaborating and engaging a broad range of stakeholders both locally and nationally” [Reference Kon5]. The UIC CCTS convenes the Community Engagement Advisory Board (CEAB) to provide consultations to investigators at UIC. The board contains a mix of community members, representatives from community organizations, and university representatives. The CEAB responds to the recommendation of the Institute of Medicine’s 2013 report on the NIH CTSA program, names CTSA funded institutions should, “involve patients, family members, healthcare providers, and other community partners in all phases of the work of the CTSA” [Reference Liverman24]. Board meetings occur 8 times per year. At a typical meeting, 2 researchers or research teams give a brief presentation to the board, with specific questions asked of the board to provide feedback. They often seek feedback on improving community outreach, refining marketing materials, boosting retention, and similar goals for individual studies. Because researchers have presented on a variety of studies, CEAB members are familiar with a range of research occurring at UIC, making them the logical choice to consider the patient impacts of institution-wide changes.
Procedures
The CEAB’s regularly scheduled June 2017 meeting served as the setting for the discussion on patient engagement. In total, 16 CEAB members participated in the focus groups. CEAB members who participated in the focus group (n=16) were evenly divided between community (n=8) and university affiliations (n=8). The majority of participants were female (n=10). The racial/ethnicity breakdown of the participants were n=8 African-Americans, n=7 Whites, and n=1 Latino. Two experienced qualitative researchers and directors of the CCTS Recruitment, Retention, and Community Engagement Program (A.K.M. and A.C.) moderated the discussion. Two staff members took notes. The discussion followed established focus group methodology [Reference Patton25]. This included using trained facilitators, 2 trained note takers, established techniques for building rapport and group interaction, and facilitator debriefing to highlight important findings. After everyone introduced themselves, one of the facilitators (A.K.M.) lead discussions about each method, following a similar format each time. First, a brief overview of the method was presented. This was followed by an opportunity for CEAB members to ask clarifying questions. CEAB members were then asked to provide initial impressions about the recruitment approach, discuss possible benefits, potential concerns, and to identify other potential problems with implementation. Then, members filled out survey questions related to that specific method. After discussing all of the methods, participants were then asked to provide overall thoughts. When available, CEAB members were presented with data from other universities that had implemented the method being discussed (i.e., universal consent enrollment or passive registry recruitment). The final section of the survey asked CEAB members to rank the approaches in terms of perceived acceptability to UIC patients. All procedures were approved by the institutional review board of the UIC.
Survey Data
A brief survey was developed to obtain individual level feedback from participants. For each approach, participants answered questions about perceived acceptability to patients, whether they would recommend this approach for use at our institution, and anticipated benefits and barriers to each approach. Where appropriate, participants were also asked to provide feedback on issues related to implementation including response options for consenting, location of registries and whether staff supported would be needed to assist patients.
Data Analysis
Immediately after the group ended, staff members and a community advisory met to debrief about the session. Later, staff members compiled their notes and compared them to ensure agreement. One staff member entered survey data and responses to open-ended questions into a database; another staff member completed a quality assurance check. Frequencies, means, and standard deviations were used to summarize the survey data using SPSS 24. The small sample size prevented more advanced statistical analysis. Two raters reviewed the notes and qualitative survey responses for key themes. While keeping the original evaluation questions in mind, qualitative data were coded and categorized into themes. Coding categories were then used to summarize key ideas in the focus group as described by Stewart and Shamdasani [Reference Stewart and Shamdasani26].
Results
Survey Results
Patient Registries
Figs. 1–3 display participants’ acceptability and recommendation ratings for each of the 3 patient engagement approaches presented. As shown in Fig. 1, 93.8% of CEAB members felt that patient registries would be acceptable to patients. Mean initial ratings for likelihood of recommending patient registries for implementation at UIC were M=8.73 (1=not at all likely to 10=very likely). Feedback on implementation of patient registries was mixed: 46.2% (n=6) of participants indicated the registries should be located in clinics and registration completed with staff assistance, 41.7% (n=5) preferred patients register at home on their own computer, and 8.3% (n=1) preferred a stand-alone kiosk in the clinical setting with no staff support (missing data n=4). When asked to rank order their recommendation for which of the 3 patient engagement approaches to adopt at our institution, 77% of participants ranked patient registries as a first or second choice.
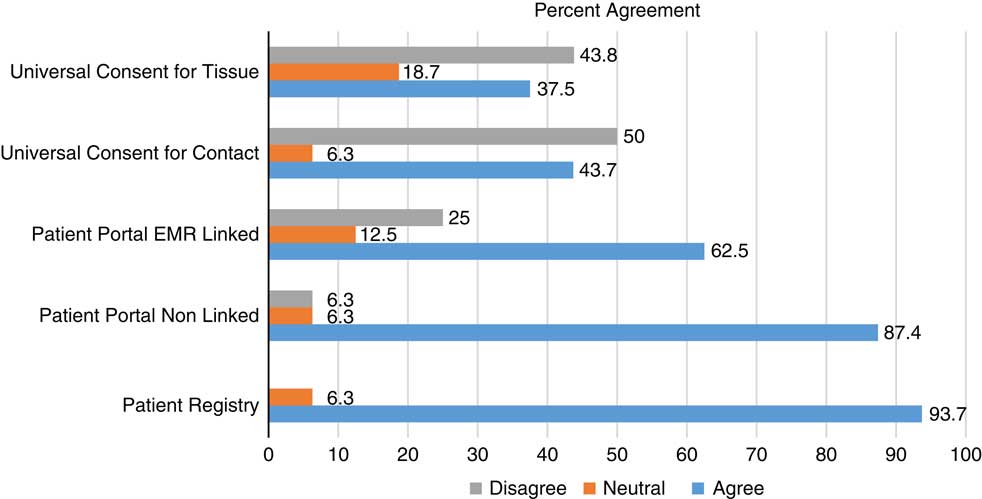
Fig. 1 Ratings of perceived acceptability of each patient engagement approach (n=16).
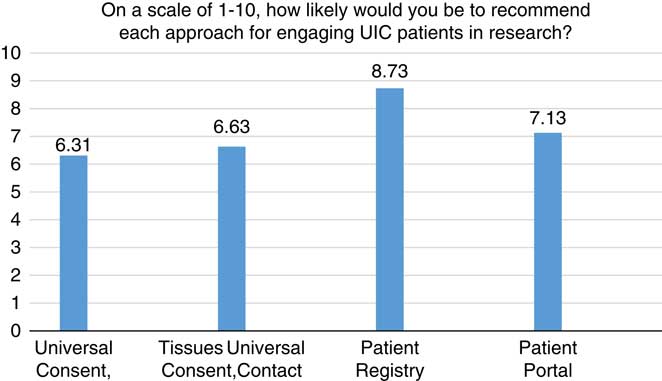
Fig. 2 Participant ratings of preferred patient engagement approach.
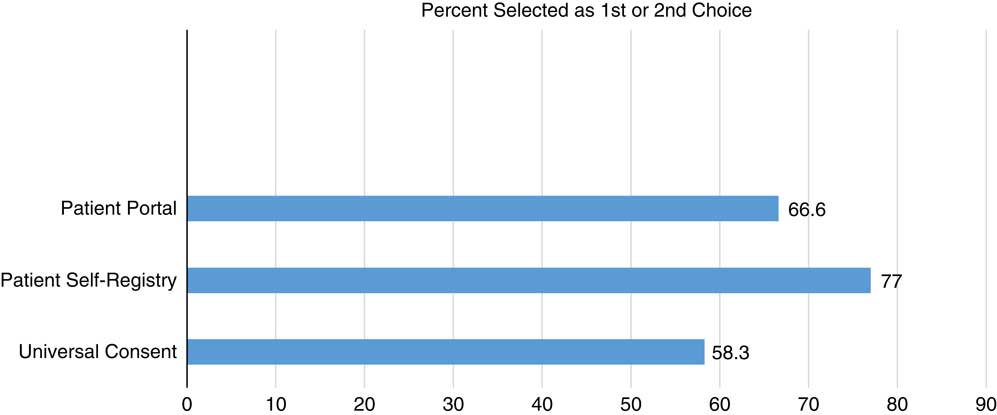
Fig. 3 Participant rankings of preferred patient engagement approaches.
Patient Portals
CEAB members were asked to reflect on 2 types of patient portal approaches: the use of patient portals to provide patients with information about research opportunities in general and the use of patient portals that use EMR data to notify patients about specific studies for which they are eligibility based on demographic or clinical data. In total, 87% of participants felt that patient portals that were not linked to their EMR would be acceptable to most patients. Perceived acceptability ratings dropped to 63% when considering EMR linked patient portals. Mean initial scores for whether they would likely recommend patient portals for implementation were 7.13. When asked to rank order their final recommendation for which patient engagement approach to adopt, 66.6% selected patient portals as a first or second choice.
Universal Consent
Universal consent was perceived to be the least acceptable to patients. In all, 44% of CEAB members felt that universal consent for contact for future research would be acceptable to patients. Acceptability ratings were even lower for universal consent to use residual specimens (37%). Mean scores for recommendation of implementation of universal consent for follow-up contact were M=6.63 and universal consent for residual specimen use were M=6.31. After being shown data indicating that a majority of patients in a study by the University of Washington signed up for universal consent, the mean support for it did rise, but only to 6.92. CEAB members were also asked about how to phrase the response options for contact for future research. The majority (63%) of respondents favored an answer set of yes, no, no-not at this time, and no-do not ask me ever again. Finally, 58.3% of CEAB members rank ordered universal consent as their first or second choice for implementation at UIC.
Qualitative Findings
Patient Registry
Qualitative feedback regarding patient registries was largely supportive and consistent with the quantitative data. CEAB members identified several positive aspects of patient registries. Members felt that compared to universal consent for research participation, patient registries would be easier to administer, would potentially involve less staff time, would be nonintrusive, and that patient decision-making would not occur in stressful situations such as at the time of a hospital admission or medical procedure. Members could also foresee ample opportunities to increase patient awareness and understanding of voluntary enrollment in the registry databases. This could take the form of informational videos playing in the waiting rooms, handouts given to patients and/or building out the registry website to educate patients considering joining the registry. Concerns regarding this approach mainly focused on issues of equity and use: that participation rates among marginalized communities and people with limited literacy may be lower, that staff support would be needed to help people navigate the registry, and that fewer people overall might enroll compared with universal consent approaches. However, some participants worried that registries could be used to sign up a family member without their consent. Finally, issues associated with “technology gaps” associated with age, English-language skills or computer literacy were raised as potential barriers to achieving a representative pool of volunteers. The use of lay health workers and research navigators were viewed as low-cost and effective ways to educate patients and assist them with self-registration.
Patient Portal
The presentation of patient portals for increasing research participation generated much discussion. The members were least familiar with this concept and as such had many questions about the method, particularly communication. CEAB members wondered if there would be an email or alert letting patients know when they had been identified as possibly eligible to participate in a study, especially if a study was time sensitive. However, they suggested the frequency of community should be controlled, either by automatics settings with in app or user-defined settings.
Despite having numerous questions about the approach, CEAB members identified many pros associated with patient portals. Members felt it would be relatively easy for patients who were already using the portal to enroll in the research registry. They noted the confidentiality of the approach and that patients could consider whether to participate in a study without pressure and over time. Another element of patient portals that members were supportive of was the potential for individualizing the information about research opportunities that they receive. For example, patients could express interest in noninvasive studies only or studies that were related to a specific disease or disorder. The possibility for patients to establish their preferences was seen as increasing a patients’ autonomy and likelihood of participating in a specific study. Further, participants felt that this approach had the potential to increase research literacy by offering a section on frequently asked questions or a help-line option where they could talk to a staff person. Despite the enthusiasm, CEAB members identified disadvantages, especially related to access. Many were concerned that using the portal would exclude certain groups: racial and ethnic minorities, people with lower reading literacy, and people with lower technological savvy (which they assumed to be older patients). As a result, they feared there would be fewer overall patients, and a less diverse patient pool, compared with other approaches.
Universal Consent
Qualitative feedback provided by CEAB members regarding universal consent approaches helped to illuminate issues related to the lower perceived acceptability scores. In general, CEAB members saw universal consent approaches as mainly benefitting the university and the hospital for example by increasing access to materials for researchers, at a relatively low cost. The questions CEAB members asked during the discussion about universal consent for tissue samples reflected a concern for patients’ rights, including how the samples would be used, who would “own” the samples, and what rights patients have to be informed about what happens to their tissue. In addition to concerns about privacy, numerous anticipated barriers to patient acceptability of universal consent for residual samples included patient health literacy, lack of trust in the healthcare system, and the likelihood that patients would be approached for consent just prior to a stressful medical procedure.
Unlike universal consent for residual tissue, universal consent to be contacted for research was perceived to have benefits for both patients and the institution. Perceived benefits included a greater pool of patients from which to draw, increased participation among underrepresented populations, and ease of use for both researchers and patients. Questions that were raised about the approach included how often patients would be contacted and by whom (how would it be determined which investigators inside the institution would have access to the database; would access be given to investigators outside of the institution), and what protections would be put in place to ensure patients confidentiality. A primary concern identified by CEAB members was how to phrase the opt-in choices on the consent forms. Allowing participants to completely opt-out of research contact was deemed as extremely important among CEAB members to increase acceptability and reduce dissatisfaction among patients. As such, the consensus among the group was that options on the consent form should include: “Yes,” “No-not at this time,” and “No-do not ask again.”
Discussion
The purpose of this study was to obtain community stakeholder input on strategies proposed by a CTSA to increase the research participation of patients receiving healthcare at a large urban hospital. Members of a CEAB of the UIC CTSA provided feedback to help guide institutional decision-making wide-reaching approaches to patient research engagement. Each of the three proposed strategies was viewed as having merit. Patient registries were viewed most favorably by participants followed by patient portals and universal consent for research forms that could be signed (or not) by all patients receiving care in the healthcare system. Patient registries were thought to have the potential to reduce or eliminate many of the potential patient concerns associated with the other 2 approaches including being less intrusive, less stress inducing, and providing more opportunities for patient education. Participants also expressed enthusiasm for patient portals as these were seen as giving patients control over identifying the types of research in which they would like to participate. In addition, it was thought that patients who initiated registration would be more likely to participate compared with a patient who responded to a request for universal consent to participate in research. However, universal consent was seen as having the potential to reach the largest number of patients but was thought to increase the risk of patient distress, back-lash and overall lower yields due to “passive refusal rates.”
Regardless of the method chosen, trust and education were identified as integral to any effort on the part of the university healthcare system to increase research participation. Similarly, board members suggested further research on patient experiences and practices with the proposed patient engagement approaches including the proportion and characteristics of patient volunteers who actually enroll in future research studies. Some data on use and enrollment in hospital-based patient engagement strategies are starting to emerge [Reference Bryant11]. However, more extensive developmental and pilot research are needed to better understand patient preferences, use experiences, and consumer-driven recommendations for maximizing patient acceptability. There is also need for additional research aimed at the development of understandable, salient and motivating educational materials for patients pertaining to research participation in general and use of specific patient engagement approaches more specifically. Although the CEAB members were asked to reflect on patient engagement approaches within the environment of hospitals and outpatient clinics, they also felt these approaches were appropriate for community health settings as well.
Limitations
Our study included a small sample of the target population from a single geographical location, thus additional studies are required. Study participants were recruited based on their involvement with the CTSA CEAB and thus may not apply to general groups of community members. Although generalizability is not a goal of qualitative research, additional research with community members with less comfort and familiarity with hospital-based clinical research activities is needed.
Conclusions
The study findings contribute to the extant literature on clinical research recruitment by engaging a community advisory board to provide input on the relative level of acceptability of each of the 3 types of patient recruitment approaches including potential strengths and barriers. This information can be used to inform future research on the selection of patient engagement approaches.
Acknowledgments
The authors would like to acknowledge and thank the members of the Community Engagement Advisory Board of the University of Illinois Center for Clinical and Translational Sciences for their expertise and commitment to improving community engagement in research.
Funding
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS), Grant Number UL1TR002003 (Mermelstein/Tobaccman, PIs). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Cancer Institute.
Disclosures
The authors have no conflicts of interest to declare.








