Introduction
The pink bollworm Pectinophora gossypiella (Saund.) (Lepidoptera: Gelechiidae) is one of the most important pests of cotton and is distributed throughout the world's cotton-growing areas (Pearson, Reference Pearson1958). The bollworm lays its eggs on squares, flowers or green bolls. Its destructive larval stage is usually buried within the cotton's fruiting bodies, unreachable by insecticidal sprays. Larvae feed on squares, flowers and bolls, including the seeds within bolls. Damage to squares and flowers can be substantial if infestation occurs in the early- and mid-growing season (Hari Prasad, Reference Hari Prasad1999). They web the cotton flower petals, imparting a characteristic ‘rosette’ appearance. Feeding within the boll results in malformation, rotting, premature or partial boll opening, reduction in fibre length and overall reduced quality of the cotton crop due to staining of the lint (Ingram, Reference Ingram, Matthews and Tugstell1995).
Bollgard™, a transgenic Bt cotton, was approved for commercial cultivation in India in 2002. It protects against the primary bollworm complex comprising the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae), the spotted bollworm Earias vittella (Lepidoptera: Noctuidae) and the pink bollworm P. gossypiella. Bollgard II™, a two-gene Bt cotton, was approved for commercialization in India in 2006. During the 2008 growing season, Bt cotton was grown on a total of 7.6 million ha in the cotton belts of north, central and south India (James, Reference James2008) constituting 79% of the total cotton area (9.6 million ha) in India. In 2008, some 158 single-gene and 63 two-gene Bt cotton hybrids belonging to three Bt cotton technology providers were available on the market in India.
As a resistance management measure, the susceptibility of multiple geographical populations of H. armigera and E. vittella was studied for 2 years before the commercial approval of Bt cotton in India. Resistance monitoring in H. armigera is conducted annually (Kranthi and Kranthi, Reference Kranthi and Kranthi2008). However, similar studies on P. gossypiella have not been carried out in India, primarily because of difficulties with its mass rearing in the laboratory.
The study reported here had two aims: (1) to develop a mass-rearing diet for P. gossypiella using locally available ingredients and (2) to optimize the bioassay for testing Bt proteins against the bollworm.
The test diets used some of the ingredients tested by previous researchers (Adkisson et al., Reference Adkisson, Vanderzant, Bull and Allison1960; Raulston, Reference Raulston1971; Patana, Reference Patana1977; Navarajan Paul et al., Reference Navarajan Paul, Parshad and Gautam1987), and bollworm development was compared with that on commercial diet premixes obtained from the USA.
Materials and methods
Starter culture
A field culture of P. gossypiella (around 500 larvae) was obtained from mature or open bolls collected from infested non-transgenic cotton fields within the Dharwad district in India. The larvae were fed on green bolls maintained in plastic trays in an environmental chamber at 27 ± 1 °C, RH 60 ± 5% and 9 h light–15 h dark photoperiod until pupation. Healthy pupae were sexed and stored in plastic vials (4 cm diameter and 5 cm height) with a filter paper disc at the bottom and a lid with a mesh window. These vials were retained in an incubator at 28 ± 1 °C until adult emergence.
Rearing
Twenty-five pairs of adult P. gossypiella were released into one oviposition jar (transparent plastic container of 28 cm height and 24 cm diameter). Adults were fed on a mixture of ‘Proteinex’ (a protein supplement), honey and distilled water (1:1:3 ratio) delivered in a ball of absorbent cotton placed in a small glass dish inside the oviposition jar. Another cotton ball dipped in distilled water was also placed in each jar. Each jar also contained a small twig of conventional cotton bearing a few leaves and squares, which served as a substratum for adults to rest and oviposit. The cut end of the twig was kept in water and inserted through a hole in the cap of a small plastic vial. The opening of the oviposition jar was covered with white cotton cloth fastened with rubber bands. Oviposition jars were maintained in an environmental chamber and adults were transferred to a fresh jar every third day. Moths laid eggs in leaf axils, on the squares and on the ventral surface of the leaves. Eggs were transferred to plastic vials (5 cm height and 4 cm diameter) with plastic mesh lids. These vials were placed in an incubator at 28 ± 1 °C. Vials were examined daily and neonates were transferred to experimental diets using a fine hair brush.
Experimental diets
Two commercial diet premixes for rearing PBW were imported from the USA, and the development of P. gossypiella on the premixes was compared with that on the test diets formulated in our laboratory. Details of the diets used are given in Table 1.
Table 1 Experimental and commercially available diets used for mass rearing of the pink bollworm Pectinophora gossypiella
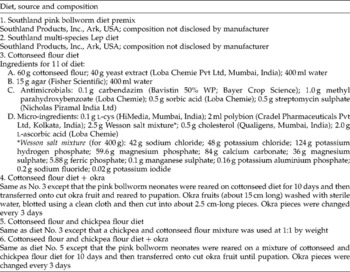
Prepared hot diet was poured into large glass Petri plates (19 cm diameter) to a thickness of 1 cm and allowed to cool and solidify. The solidified diet was diced into 1 cm3 cubes and a cube was placed in each of the 32 wells of an insect culture tray (CD International, Pitman, NJ, USA). Freshly emerged P. gossypiella neonates were released onto diet cubes at 1 neonate/well and reared until pupation.
Wherever okra was used as the second-phase diet, 10-day-old larvae were transferred to slices of okra (Table 1). Pupae were sexed (Navarajan Paul et al., Reference Navarajan Paul, Ram and Parshad1979), weighed and kept individually in plastic vials until adult emergence.
Observations
Larval period, pupal weight, pupal period, generation time, pupal malformation, adult emergence, adult malformation, adult longevity and sex ratio were recorded for each diet. One-way ANOVA in Microsoft Excel was used to compare the performance of the bollworm stages on the different diets.
Bioassay with Bt protein Cry1Ac
A freeze-dried commercial formulation of MVP-II® (Cell-Cap® encapsulation system by Mycogen, San Diego, California, USA) was used as the source of Cry1Ac protein. The formulation contained 19.7% (w/w) of Cry1Ac protein, as determined using the diet-incorporation method (Sims et al., Reference Sims, Greenplate, Stone, Caprio and Gould1996). A primary stock solution for Cry1Ac was prepared by vortexing 12.69 mg MVP-II powder in 10 ml of 0.2% agar solution. Seven serial dilutions were prepared in sterile water in centrifuge tubes (50 ml) using threefold dilution.
Southland multi-species Lep diet™ was prepared in sterile glass bottles and kept in a water bath at 60 °C. The diluted Cry1Ac standards (3.28 ml) in each of the serial dilution tubes were thoroughly vortexed with 13.12 ml of warm diet (60 °C) and approximately 1 ml was poured into each well of insect bioassay trays (CD International Trays™, MA, USA). The final concentrations of Cry1Ac were 1.5, 0.5, 0.167, 0.056, 0.019, 0.006 and 0.002 μg/ml of diet. The diet trays were allowed to dry for 45 min.
Active P. gossypiella neonates (1 neonate/well) were transferred onto the solid diet with a fine hair brush on a laminar flow clean air bench. Bioassay trays were then covered with self-adhesive Pull-N-Peel™ tabs (CD International) and transferred to an incubator maintained at 27 ± 1 °C. Sixteen larvae were exposed to each of the various Cry1Ac concentrations and an untreated control. The entire assay was replicated 10 times.
Bioassay with Bt protein Cry2Ab2
The source of Cry2Ab2 protein was leaf powder from transgenic maize plants (event MON 84 006) containing Cry2Ab2 protein (3 mg/g of corn leaf powder). The Cry2Ab2 protein produced in transgenic maize leaves is a very stable source of Cry2Ab2 protein, and has been used in bioassays and resistance monitoring studies globally. Assay methods and the test arena were identical to those used in the Cry1Ac assays.
The primary stock solution for Cry2Ab2 was prepared by vortexing 5.2 mg maize leaf powder in 125 μl of sterile water. Seven serial dilutions were prepared in sterile water in centrifuge tubes (50 ml capacity) using twofold dilutions. Diluted Cry2Ab2 standard remaining in each of the serial dilution tubes (3.28 ml) was vortexed with 13.12 ml of warm diet (60 °C), and approximately 1 ml of the mixture was poured into each well of the insect bioassay trays. The final concentrations of Cry2Ab2 in diet were 0.2, 0.1, 0.05, 0.025, 0.013, 0.006 and 0.003 μg/ml of diet. Sixteen larvae were exposed to each of the various Cry2Ab2 concentrations and an untreated control. The entire assay was replicated 10 times using neonates that had hatched within 4 h.
Observations for Bt assays
Mortality, instar (determined from head capsule size) and group weight of surviving larvae were recorded 21 days after infestation (Tabashnik et al., Reference Tabashnik, Patin, Dennehy, Liu, Carrière, Sims and Antilla2000). Probit analysis, carried out using SPSS software (SPSS Inc., Chicago, Illinois, USA), was used to calculate:
lethal concentrations (LC50 and LC90),
moult-inhibitory concentration (MIC) = concentration of protein that severely limited larvae from developing beyond the first instar, and
inhibitory concentration (IC) = concentration of protein that prevented larvae from reaching the third instar.
Log-linear regression analysis of the data using JMP software (SAS Institute Inc., Cary, North Carolina, USA) was used to calculate:
effective concentration (EC) = concentration of protein that reduced larval weight by a certain percentage relative to untreated controls.
The above concentration–response parameters were expressed in μg of Cry1Ac or Cry2Ab2/ml of diet.
Results and discussion
Growth and development on various diets
Growth and development parameters for P. gossypiella on the six different diets are presented in Table 2.
Table 2 Growth and development of Pectinophora gossypiella on various diets
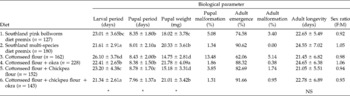
*Significant difference at P = 0.05; NS, no significant differences.
Larval period (five instars) was shortest on a diet of cottonseed/chickpea flours + okra (21.34 ± 2.61 days); this period was comparable to that for bollworm larvae reared on the Southland multi-species (SM) diet. The shortest pupal period (7.96 ± 1.37 days) was observed on cottonseed/chickpea flours + okra. As such, P. gossypiella completed its life cycle fastest on a cottonseed/chickpea flours + okra diet. Larval and pupal periods were the longest on sole cottonseed flour diet, which gave the slowest overall development (34.53 ± 4.49 days).
Pupal weight, an indicator of food conversion efficiency during larval stages, was highest on the cottonseed flour + okra diet (21.78 ± 4.09 g). Pupal weights reached with cottonseed and chickpea flours + okra and SM diets were not significantly different (P = 0.05).
Pupal malformation was high when P. gossypiella was reared solely on cottonseed flour diet (13.48%), but decreased to 1.86% when larvae reared on cottonseed flour were transferred to okra fruit slices after 10 days. Pupal malformation was lowest (1.31%) on cottonseed/chickpea flours+ okra diet.
Maximum adult emergence (91.66%) was recorded on the cottonseed/chickpea flours + okra diet. Adult emergence was greater when larvae were reared on cottonseed flour diet followed by okra (88.32%), compared with a sole cottonseed flour diet (62.06%). Five per cent of emerged adults were malformed on cottonseed flour diet, but this was reduced to 0.38% when 10-day-old larvae reared on cottonseed flour were transferred to okra.
It appears that the transfer of 10-day-old larvae from the semi-synthetic diet to cut okra pieces significantly decreased pupal malformation and improved normal adult emergence. This is likely because okra is a natural diet of the insect. In the mass rearing of P. gossypiella using the two-step procedure, it is important that the okra has no insecticide residue as such residues can contribute to retarded growth and malformation at the pupal or adult stages.
Adult longevity ranged from 21.05 to 24.65 days, but did not vary significantly across the diets tested. Adult sex ratio was close to 1:1 on all the different diets.
Our results showing superior larval development of P. gossypiella on a diet of cottonseed and chickpea flours when compared with a sole cottonseed flour diet corroborate a study by Navarajan Paul et al. (Reference Navarajan Paul, Parshad and Gautam1987), which recommended that P. gossypiella diet should contain chickpea flour and cottonseed flours in a ratio of 1:1.4. Similarly, Hari Prasad (Reference Hari Prasad1999) observed growth and development of P. gossypiella at par with natural diet when chickpea and cottonseed flours were used in a ratio of 1:1.4. However, fungal contamination was a frequently encountered problem with our experimental diets. Such contamination was minimal on the commercial SM diet, and for this reason commercial diet was used for evaluating the sensitivity of the bollworm to Cry1Ac and Cry2Ab2 proteins.
Sensitivity of P. gossypiella to Cry1Ac and Cry2Ab2 proteins
P. gossypiella neonates were extremely susceptible to Cry1Ac and Cry2Ab2 proteins, as shown by the low lethal concentration (LC), moult-inhibitory concentration (MIC) and inhibitory concentration (IC) values recorded (Tables 3 and 4, respectively).
Table 3 Concentration-dependent response of Pectinophora gossypiella neonates to Cry1Ac protein
Values are expressed as μg Cry1Ac/ml of diet (ppm); n = 160 (total number of P. gossypiella neonates exposed to a single concentration of Cry1Ac); no. of graded concentrations per assay = 7; no. of assays = 10. NA, not available; χ2: P ≤ 0.017.
Table 4 Concentration-dependent response of Pectinophora gossypiella neonates to Cry2Ab2 protein

Values are expressed as μg Cry2Ab2/ml of diet (ppm); n = 160 (total number of P. gossypiella neonates exposed to a single concentration of Cry2Ab2); no. of graded concentrations per assay = 7; no. of assays = 10. NA, not available; χ2: P ≤ 0.018.
At the end of the 21-day assay period, some P. gossypiella larvae exposed to Cry1Ac or Cry2Ab2 survived, but almost all survivors were still in their first instar, and were severely stunted, inactive and weak. These surviving larvae were seen surviving on water condensed on the inner wall of the bioassay tray wells. Because of this characteristic of pink bollworm larvae, it is advisable to use MIC values rather than LC values to evaluate pink bollworm tolerance to Bt insecticidal proteins.
Overall, these results indicate that Indian P. gossypiella is extremely sensitive to the Bt proteins contained in the transgenic cotton varieties Bollgard and Bollgard II. Baseline studies using the same insecticidal proteins should be conducted with various additional field-collected Indian bollworm populations.
Conclusion
For the mass culturing of P. gossypiella, a two-step diet was found to be optimal. The first phase consisted of rearing the larvae on a semi-synthetic diet composed of cotton and chickpea flours as the main ingredients for 10 days from emergence, and then on cut okra pieces until pupation. This two-phase diet is made from locally available ingredients and the methodology can be easily adopted by Indian researchers, saving them the importation of expensive diet premixes. This is the first report of using such a diet combination to successfully culture the pink bollworm in the laboratory. A laboratory colony established from field-collected P. gossypiella has now been reared for 51 generations on this diet.
This study also found that P. gossypiella neonates were highly susceptible to the Bt proteins Cry1Ac and Cry2Ab2.
Acknowledgements
The authors acknowledge the support received from the members of Biotech Product Support Team of Monsanto Research Centre, Bangalore, during the field collection of P. gossypiella, laboratory rearing and conduct of bioassays. The authors also thank Professor Tim Dennehy for his technical inputs and review of the manuscript, and Dr G.C. Unnithan for his advice on insect rearing.








