Lack of evidence is one of the major obstacles to ensuring timely access to new medical technologies. Decisions on their adoption or coverage by any health care system should be based on sufficient and reproducible data. “Coverage with Evidence Development” (CED) is an approach that aims to bridge this gap and to allow for early adoption of pharmaceuticals, medical procedures and medical devices for a limited period of time, and under explicit restricting conditions. This policy is applied if novel medical technologies are promising yet controversial or where additional evidence is required to make an informed decision. One of the most interesting aspects of CED is that it integrates HTA into decision making, allowing direct “implementation” of HTA results into the technology appraisal process.
The name CED was coined in the United States in 2005 by the Centers for Medicare and Medicaid Services (CMS) (Reference Tunis and Pearson1). The concept as such, however, existed before this and can vary according to country or reimbursement system. It carries different names in other jurisdictions such as “Only in Research” (United Kingdom), “Still in Research” (France), “Interim Funding” (Australia), “Conditionally Funded Field Evaluation” (Ontario), or “Monitored Use” (Spain) (2). In 2008 the European network for Health Technology Assessment (EUnetHTA) developed a special toolkit for monitoring the development of emerging technologies and refers to these mechanisms as “Access with Evidence Generation.”
Principles of good practice for CED were developed at the so-called Banff Summit and published as a consensus statement. They call for transparency, clarity, scientific rigor, appropriateness and independence from vested interests (Reference Menon, McCabe, Stafinski and Edlin3). Academic interest to better understand the functioning and the implications of CED has grown in line with the dissemination of the concept (Reference Stafinski, McCabe and Menon4;Reference Mohr and Tunis5). Incidentally, a clear sign of the relevance of the subject is the establishment of a special “Interest Sub-Group on Conditional Coverage and Evidence Development for Promising Technologies” by the international HTA organization HTAi, with 250 members at the end of 2012.
Switzerland, where this concept was adopted by the Federal Office of Public Health (FOPH; previously Office of Social Security) as early as 1996 (6), presents a particularly remarkable example of CED in the area of nondrug technologies.
The documentation accompanying the application for coverage of new and controversial technologies has been supported and regulated since then in a series of further guidelines (7). However, little is known about the implementation and the outcome of the Swiss program.
Regulation of CED for Medical Procedures in Switzerland
Since 1996, when a new Federal Law on Sickness Insurance came into force, all residents have been covered by basic health insurance. In the Procedures Ordinance of January 1st 1996, the insurance scheme accepted for the first time a series of medical technologies for coverage which were novel and promising, but for which existing evidence was incomplete. Among the first technologies denoted “in evaluation” were five different technologies for the measurement of mineral bone density within a multi-center study. The decision “Yes, in evaluation” made the procedure obligatory for reimbursement by the health insurers.
In principle, all medical services and procedures that are effective, appropriate and cost-effective are covered by social health insurance in Switzerland. This means that Swiss physicians and hospitals enjoy the so-called “principle of trust” unless challenged by anyone with a legitimate interest, for example, a health insurance provider. In such a case, the contested medical service or procedure goes through an HTA process and its outcomes are published in the appendix of the relevant procedures ordinance. Thus, there is only a negative and conditional list for medical services and procedures (including diagnostics and devices) in place.
A potentially controversial medical service has to be reported to the Federal Office of Public Health (FOPH), which is responsible for, among other things, the supervision of the benefit basket. In a first step, the FOPH consults the Swiss health insurer's association and the Swiss medical association. If this first rough assessment of a medical service by the FOPH judges it to be undisputed, it is reimbursed. Otherwise, it goes into a structured technology appraisal process (8).
In this latter case, the provider or the manufacturer has to submit full documentation of the available evidence on effectiveness (including a systematic review), appropriateness and cost-effectiveness for obligatory coverage of the specific medical technology under the statutory health insurance scheme. The FOPH then checks the submission for completeness and writes a summary including critical issues. All information on the case is assembled in a dossier which is then handed over to the Federal Commission for Medical Benefits and Principles (FCMBP/ELGK) for appraisal.
The commission consists of eighteen representatives of health insurers, the medical profession, patient organizations, hospitals, industry, ethics etc., and is appointed by the Federal Council (Swiss government). Its mandate is to examine the effectiveness, appropriateness and efficiency of the controversial procedure and to generate a substantiated recommendation for the decision-making body, which is the Federal Department of Home Affairs. The recommendation or decision can be one of four categories: “Yes” or “No,” “Yes, in evaluation” or “No, in evaluation” (the latter option was discontinued after 2004). The decision “Yes, in evaluation” can be linked to restrictions to specific centers or medical specialists, to specific indications, or can presuppose additional requirements. The reimbursement can be combined with the obligation to keep a patient-based registry.
“Yes” and “Yes, in evaluation” mean obligatory coverage of the procedure, whereas “No” and “No, in evaluation” mean no reimbursement, and further evidence development will not be compensated. The discussions within the appraisal commission are not made public. Only the final decisions and the conditions for approval are published in the appendix of the Procedures Ordinance, which is updated at least annually.
This study aims to pursue the number and type of technologies accepted under restraint in Switzerland in the 17 years from 1996 to 2012, and to examine the duration of evaluation and its relation to the final decision. It further aims to analyze the process of decision making in the course of adoption or refusal of technologies under conditional approval. Finally, it intends to present the type and frequency of restrictions related to technologies under evaluation with special emphasis on the outcome of explicitly required registries for selected procedures.
METHODS
Study Design
This is a retrospective analysis of all controversial health technologies submitted for decisions by the Department of Home Affairs from 1996 to 2012. They were grouped according to the four categories “yes,” “yes, in evaluation,” “no, in evaluation,” or “no”; specific focus was placed on decisions for reimbursement under constraints.
Data Collection
All information was obtained from the appendix of the Procedures Ordinance, which contains the list of all controversial health technologies since 1996. The Procedures Ordinance is updated annually.
For all cases with “yes, in evaluation” and “no, in evaluation” additional information was collected: type of technology, date of initial decision, end and duration of evaluation period, prolongation of the evaluation period if applicable, specific conditions of evaluation if applicable, and final decision.
An in-depth analysis was performed for two technologies where registries were required: spine surgery and hematopoietic stem cell transplantation.
Statistical Analysis
Descriptive statistics were used. Time to evaluation was assessed with modified cumulative incidence plots. In other words, the probabilities of the two potential final outcomes (“yes” / “no”) are displayed in two cumulative incidence functions over the duration of evaluation. To improve the interpretability, the incidence function for a “final no” has been replaced by one minus the probability of a “final no.” Hence, the difference between these two curves represents the probability that the decision maker has not yet come to a final decision, that is, these cases still await approval or rejection. The applied estimation method of these functions takes into consideration the fact that some of the observations are censored, and that there is a competing outcome (Reference Gooley, Leisenring, Crowley and Storer9). The computation has been done by the Package “cmprsk” (Version 2.2–2 by Bob Gray) in the R system (10). To test for homogeneity in a two-way contingency table we use Pearson's Chi-squared test as described, for example, in Agresti (Reference Agresti11). The test is performed using the function chisq.test in the R core system.
RESULTS
Use and Incidence of CED in Switzerland
A total of 120 controversial health technologies were evaluated and decided upon by the Federal Department of Home Affairs from 1996 to 2012. Of these, a total of forty-five technologies (37.5 percent) were classified “yes, in evaluation” and fell, therefore, under the regime of CED for a certain period of time (Table 1). Diagnostic procedures (n = 10) and surgical interventions (n = 7) were the most frequent technologies in the “yes, in evaluation” category. Alternative medicine holds the same position (n = 10), which might be somewhat surprising. This local peculiarity in Switzerland requires some explanation. Several methods of alternative medicine were set under the CED regime from 1999 until 2005. Based on a large evaluation program the responsible minister took a “no” decision for most of those methods at the expiry of the evaluation period. After a popular referendum in 2008 in favor of the use of complementary medicine in the Swiss health system, five methods of complementary medicine were again listed as “yes, in evaluation” again in 2012 for another 5 years. In 2017, a re-appraisal will have to be made.
Table 1. Number of Medical Technologies in CED Regime by Category
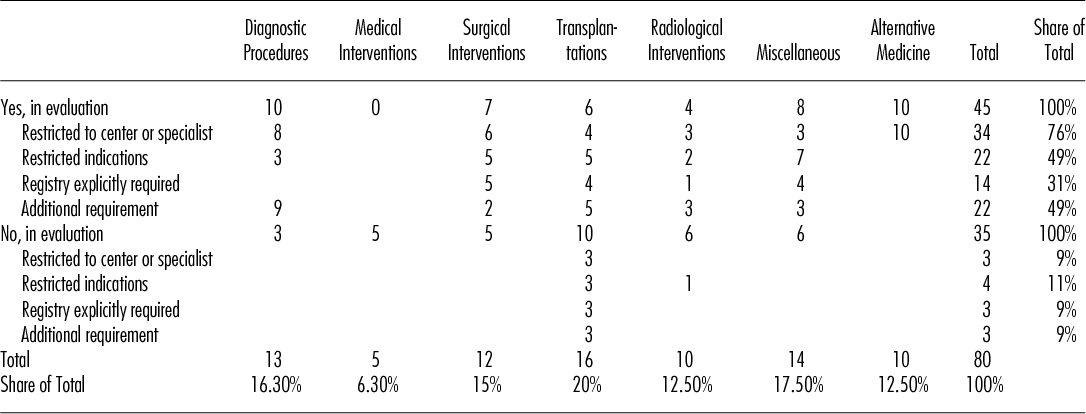
The method of CED was introduced as early as 1996 and maintained throughout the whole evaluation period. There was an apparent trend toward an increase up to the year 2003 followed by a decline. Until the year 2002, each year roughly four new technologies fell under the regime of CED (“yes, in evaluation”). From 2003 onward, this number fell to an average of just a little over one technology per year.
A total of thirty-five technologies (29.2 percent) of the 120 technologies were classified “no, in evaluation” during the same period of time (Table 1). The most frequent technologies in the “no, in evaluation” category were transplants (n = 10) followed by radiological (n = 6) interventions. This category was initially introduced to signal to the providers of those health technologies that they had a realistic chance to get reimbursement at a later stage if they could provide better evidence. Due to the fact that no reimbursement was provided for technologies in this category, it was difficult for the authorities to monitor and enforce the necessary evidence-generation. Only two of these thirty-five decisions were, upon re-evaluation, turned into “yes, in evaluation.” Both relate to hematopoietic stem cell transplants for autoimmune diseases. Thus, the category “no, in evaluation” was abandoned in 2004, although this was never officially communicated. Since that year, no new such decision has been made. Some of the technologies, however, that were classified “no, in evaluation” before that year were still listed in the appendix of the procedure ordinance with that label in the year 2013.
Restrictions
Forty-four of forty-five procedures in the CED regime are subject to further restrictions. Table 1 shows that these can be “restrictions to a center or a specialist” (34; 76 percent technologies), “restricted indications” (22; 49 percent technologies.), “registry explicitly required” (14; 31 percent technologies), or “additional requirements” (22; 49 percent technologies). Altogether, for fourteen of forty-five interventions, a registry was explicitly required. It is of interest to note that this additional request primarily related to surgical interventions (5 of 7) and transplants (4 of 6).
Outcome of Decisions “Yes, in Evaluation”
The evaluation time ranged from 1.5 years to 11 years. Mean evaluation time for the initial evaluation period without prolongation was 4.5 years (median 5 years) and 5.8 years (median 6 years) for the total evaluation period including prolongation. Figure 1 shows a longitudinal sequence of the forty-five medical technologies in the category “yes, in evaluation.” The graph also shows that the instrument was used more frequently before 2004 than thereafter.
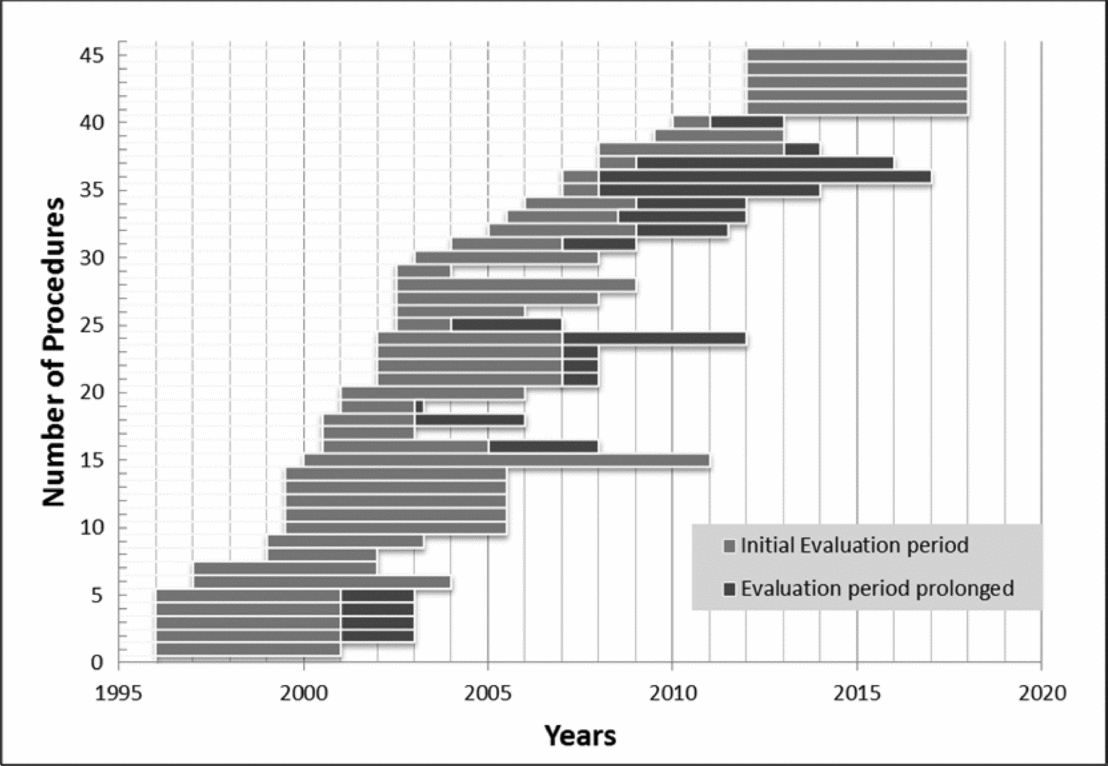
Figure 1. Longitudinal sequence of the forty-five medical technologies in the category “yes, in evaluation.”
For nineteen of the forty-five decisions (42 percent), the final outcome was “yes,” for twelve (27 percent) “no.” Three (7 percent) decisions were downgraded to “no, in evaluation.” Eleven decisions (24 percent) still remain pending. Figure 2 illustrates the two cumulative incidence functions over the evaluation time in years for the thirty-two technologies with a final decision until 2012. Most decisions are made after 3 to 7 years.
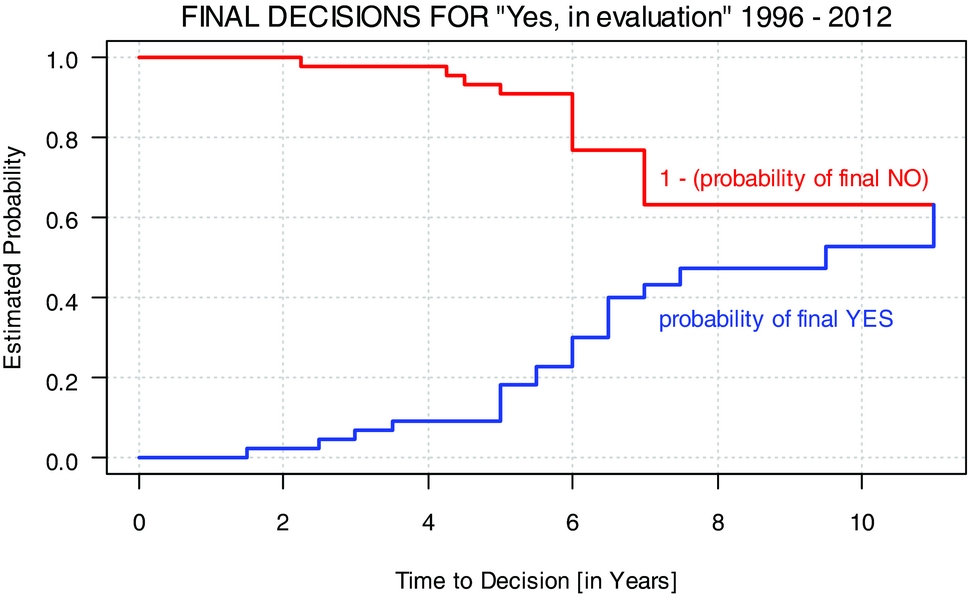
Figure 2. Final decisions for “Yes, in evaluation” 1996–2012.
Registries
Registries are one of the additional restrictions under the CED regime “yes, in evaluation.” The following examples are presented to illustrate the use of this tool in conditional coverage in Switzerland.
Example 1: Reimbursement Decisions for Allogeneic Hematopoietic Stem Cell Transplantation
Hematopoetic stem cell transplantation (HSCT) has a longstanding tradition in Switzerland and was introduced in the early 1970s when the procedure was still a challenging medical intervention (Reference Gratwohl, Brand and Niederwieser12). Still, the key indications—leukemia, aplastic anemia, and immunodeficiency—were listed in the Procedures Ordinance early on. The introduction of the new law in 1996 coincided with a large expansion of indications, donor type and stem cell source for HSCT worldwide. In contrast, scientific information on effectiveness and cost-effectiveness was lacking; the procedure was primarily driven by expectations as best illustrated by the massive transient expansion of autologous HSCT for breast cancer (Reference Passweg, Baldomero and Stern13). However, in 1996, only Lymphoma was added to the Procedures Ordinance as a novel indication with “yes, in evaluation” and the establishment of a registry was requested. The registry was established by the Swiss Transplant Working Group Blood and Marrow Transplantation STABMT and was based on the reporting structure of the European Group for Blood and Marrow Transplantation EBMT (14). It was declared mandatory for all STABMT members for all transplants, including those with a “yes” and for those congenital disorders paid for by the disability insurance and financed initially by means of the mandatory STABMT membership fee. The Procedures Ordinance was revised twice, in 2001 and 2008, with extensions of indications and changes in categories. In addition, all teams performing HSCT were obliged to fulfill a quality management program and to be accredited by the Joint Accreditation Committee ISHAGE Europe and EBMT JACIE (15). Reimbursement of the procedure was linked to JACIE accreditation. The registry was maintained. With the introduction of the Federal Transplantation Act in 2004, Swiss Blood Stem Cells obtained the official mandate in Switzerland for stem cell transplantation and has overseen the registry since then. All four teams performing allogeneic HSCT and all six teams performing autologous HSCT are currently accredited by JACIE (Reference Ljungman, Bregni and Brune16;Reference Gratwohl, Baldomero and Aljurf17) and the Swiss registry collects data on all HSCT performed in Switzerland. Outcome data are regularly published and data are shared with the EBMT and the Worldwide Network of Blood and Marrow Transplantation WBMT (Reference Passweg, Baldomero and Bargetzi18;19).
Table 2 shows the development of HSCT in Switzerland over the three different time periods. Numbers of HSCT increased over the years from 338 procedures per year in the first period to 429 in the second and to 506 HSCT per year for the period from 2008 to 2011. The proportion of procedures with a “yes” or “yes, in evaluation” increased over time from 26 percent and 22 percent respectively to 67 percent and 29 percent respectively, by contrast, the number of procedures with “no,” “no, in evaluation” and “not listed” steadily declined from a total of 50 percent to just 1 percent in the last period. Of note, approximately 2 percent of all HSCT in Switzerland, mainly for congenital disorders, are covered by the disability insurance scheme. Applying the Chi-squared test for homogeneity in the observed frequencies of the categories “yes,” “yes, in evaluation” and “no” along the three time periods, we see that the frequencies of the categories are changing over time in a statistically significant way (the value of the test statistic is 2353.9 and the corresponding p-value is smaller than 2.2e-16).
Table 2. Number of Hematopoietic Stem Cell Transplants per Time Period and Reimbursement Category
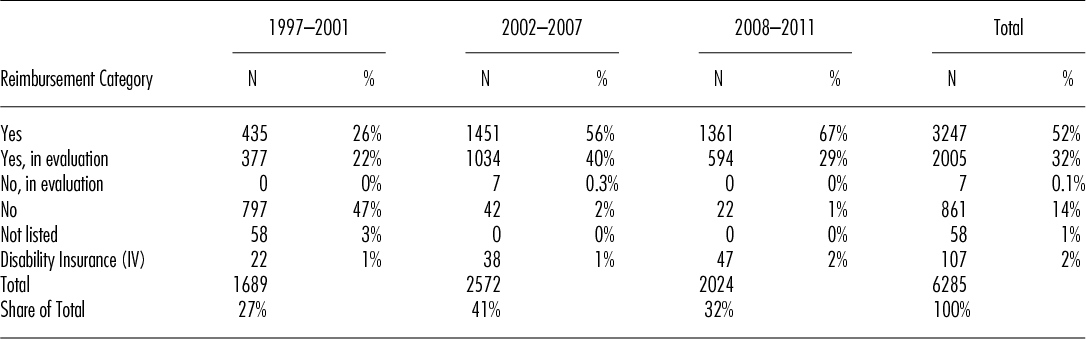
The proportion of transplants paid for by the disability insurance scheme (IV) remained stable. The massive reduction of transplants for indications with “no” is in part explained by a change in category, for example, lymphoma or plasma cell disorders, in part by renouncing certain indications, for example, renal cell carcinoma and adherence to the EBMT current practice guidelines (Reference Ljungman, Bregni and Brune16).
Example 2: Swiss Spine Registry
A second example of a mandatory registry as condition for reimbursement is orthopedic implants. Three technologies fell under the “yes, in evaluation” regime: dynamic spinal stabilization (Type Dynesis), dynamic interspinous stabilization (Type DIAM), and lumbar/cervical discs. The national registry, the so-called SWISSspine Registry, was established in 2005 at the Institute for Evaluative Research at the University of Berne and has collected data on each reported case since then. Collected items encompass back and leg pain, walking distance, consumption of medication, quality of life, and complications/revisions. No specific cost data is collected (Reference Diel, Reuss, Aghayev, Moulin and Roder20). All three technologies started with an initial evaluation period of 1 year, which was prolonged by several years in each case. The evaluation is financed by the industry, which pays a lump sum per patient of CHF 100.
The numbers of average annual collected cases were: for Dynesis 50 cases from six centers, for DIAM 149 cases from nine centers and for lumbar/cervical discs 233 cases from twenty-four centers. Results from the registry are regularly published (Reference Schluessmann, Aghayev and Staub21;Reference Aghayev, Roder, Zweig, Etter and Schwarzenbach22–Reference Roder, Chavanne, Mannion, Grob and Aebi23). No information is available as to the degree of participation in the registry for all cases with spine surgery.
DISCUSSION
Conditional coverage for medical technologies has been used in Switzerland since 1996 and been extended to a total number of forty-five such decisions of “yes, in evaluation” until 2012. Numbers were higher in the first 9 years from 1996 to 2004 (31 decisions) than thereafter (14 decisions) but the concept remains. The reasons for this apparent change are outside of the scope of this analysis; influencing factors could be a change in the respective minister, a change in the composition of the commission (ELGK) that gives the recommendation or external factors. The “no, in evaluation” category was abandoned in 2004 due to lack of influence on providers and manufacturers to generate evidence. It is of interest to note that HSCT for autoimmune diseases represents an exceptional positive example, where the “no, in evaluation” triggered research and led to prospective controlled studies.
The study clearly shows that the decisions for CED varied depending on the type of medical technology under scrutiny with a higher number of “yes, in evaluation” for alternative medicine, diagnostic procedures and surgical interventions, and a higher number of “no, in evaluation” for radiological and medical interventions or transplant procedures. The study also shows that the time from initial decision to final evaluation varies widely, independent of numbers of procedures within the technology to be evaluated or type of category. There is no obvious argument to maintain an open end for more than a decade. Again, the reasons for these patterns of decisions are unknown. The absence of explanations clearly indicates an urgent need for an evaluation of HTA evaluations.
Strengths and Weaknesses
The strengths of this study are the length of the period of time it covers and the many very different “non-drug” technologies under a CED regime as well as the concentration on a single country with a predefined HTA structure after introduction of a new law. It is the first systematic evaluation of the duration of CED decisions and final outcome for each technology. It provides information on additional criteria such as restrictions to specific centers, specialists or indications, and indicates where registries were required. Finally, the study shows data on numbers of centers and patients for two selected technologies where registries had to be kept (HSCT, Spine).
There are some clear limitations of this study. There is no information whatsoever on the reasons for the initial recommendations of the commission leading to “Yes/no, in evaluation” nor on the reasons for the final decisions “Yes/No.” There is no information on costs of these evaluations for the service provider, the health insurer or the registries (where required). There are no data from other registries, whether successful or not, nor on the reasons for success or failure. There is no information on whether the final evaluations were seen as successes or failures and for what reasons. Finally, there is no information on how far the ELGK decisions had an impact on treatment patterns in Switzerland.
CONCLUSIONS
In summary, these data clearly show that CED is a reality in Switzerland. As such, it did provide access to promising therapies early on for those cases where the final decision became a “yes”; it made it possible to base the final “no” on more solid grounds after a field evaluation. The study shows the long time span required to come to a final decision, providing an additional argument to favor CED early on in the course of introducing potentially controversial nondrug technologies. At the same time, despite a wealth of quantitative data, no information could be obtained on factors associated with final outcome. Clearly, more studies evaluating HTA decisions are warranted.
CONTACT INFORMATION
Urs Brügger (brgu@zhaw.ch), Andreas Ruckstuhl (rkst@zhaw.ch), Bruno Horisberger (horisbruno5@bluewin.ch), Alois Gratwohl (alois.gratwohl@unibas.ch)
CONFLICTS OF INTEREST
The work is the sole responsibility of the authors. There are no conflicts of interest to report.








