To the Editor—The β-lactamases are plasmid-encoded or chromosomally encoded enzymes that hydrolyze β-lactam antibiotics. Those that are plasmid-mediated can be rapidly transferred between bacterial genera and can put in check the successful use of β-lactam agents. The β-lactam/inhibitor of β-lactamase (BL/IBL) combinations are a class of agents with proven success in treating infections caused by bacteria producing β-lactamases, mostly the conventional-spectrum enzymes.Reference Payne, Cramp, Winstanley and Knowles 1
The prevalence of gram-negative bacteria resistant to broad-spectrum β-lactams has increased alarmingly in past decades, including those extended-spectrum β-lactamase (ESBL)–producing organisms with poorer clinical outcomes than more susceptible organisms.Reference Perez 2
Unequivocally, carbapenems have a relatively high clinical success rate among patients infected with ESBL-producing organisms.Reference Tamma, Han and Rock 3 However, indiscriminate carbapenem use has contributed to the increased emergence of carbapenem-resistant Enterobacteriaceae (CRE).Reference Rodrigues Perez 4
Because it is crucially important to conserve the usefulness of carbapenems in the era of antimicrobial resistance, a survey was conducted to monitor the contemporary crude prevalence of resistance rates for BL/IBL combinations against Escherichia coli, Klebsiella, and Proteus species displaying a conventional or ESBL-enzyme spectrum, including those presenting a carbapenem-resistance profile but not a carbapenemase production relation.
Enterobacterial isolates were recovered from inpatients between January 1 and December 26, 2016, at a tertiary hospital in Porto Alegre, Southern Brazil. Escherichia coli, Klebsiella, and Proteus species were selected because other minor prevalent enterobacterial species such as Enterobacter, Providencia, Serratia, and Citrobacter freundii have an intrinsic resistance to amoxicillin/clavulanate. Biochemical tests using a MicroScan automated system (Beckman Coulter, Brea, CA) were used to identify E. coli, Klebsiella, and Proteus species and to determine their resistance rates to amoxicillin/clavulanate (AMC), ampicillin/sulbactam (SAM), and piperacillin/tazobactam (TZP). All selected enterobacterial isolates were confirmed for the presence of an ESBL enzyme using a synergistic test applying clavulanic acid, as previously described.Reference Perez 2 Isolates with reduced susceptibility to any carbapenem agent were tested using a synergistic test applying phenyl-boronic acid and ethylenediaminetetraacetic acid to detect Klebsiella pneumoniae carbapenemase (KPC) and metallo-β-lactamase enzyme, in that order. Only CRE isolates with a negative result for any carbapenemase were included in this study.
A total of 942 isolates were included in this survey; 878 isolates (93.2%) had a community profile: 441 E. coli (50.2%); 213 Proteus mirabilis (24.3%); 210 K. pneumoniae (23.9%); and 14 K. oxytoca (1.6%). In addition, 62 isolates (6.6%) had an ESBL-producing spectrum: 53 K. pneumoniae (85.5%), 8 E. coli (12.9%); and 1 P. mirabilis (1.6%). Only 2 isolates (0.2%), K. pneumoniae, and E. coli, had a carbapenem-resistance profile. Of these isolates, 591 (62.7%) were recovered from urine, 174 (18.5%) were recovered from blood, 92 (9.8%) were recovered from respiratory secretions, 19 (2%) were recovered from catheter tip, and 66 (7%) were recovered from elsewhere.
Resistance rates to AMC, SAM, and TZP for each categorized group (community-based, ESBL-producing, or CRE profile) are shown in Table 1. Overall, among the BL/IBL combinations, TZP was the most active combination (14.6% of resistance rate), followed by AMC (32.3% of resistance rate) and SAM (51.9% of resistance rate). The greatest potency of activity was shown by TZP in both community-based and ESBL-spectrum profiles. None of the combinations were active in vitro against CRE isolates.
TABLE 1 Resistance Rates of BL/IBL Combinations Against Escherichia coli, Klebsiella, and Proteus Species
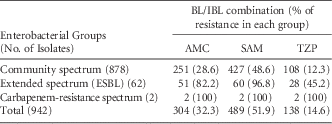
NOTE. BL/IBL, β-lactam/inhibitor of β-lactamase; AMC, amoxicillin/clavulanate; SAM, ampicillin/sulbactam; TZP, piperacillin/tazobactam; ESBL, extended-spectrum β-lactamase.
A more liberal use of carbapenems is not without consequence and may result in the emergence of a resistance to this agentReference Rodrigues Perez 4 as well as others, such as polymyxinsReference Rodrigues Perez and Dias 5 , Reference Perez 6 and fosfomycin,Reference Perez 7 due to the influence of an increased demand, which may severely limit future treatment options.
In this study, tazobactam was the most superior (while sulbactam was the less active) IBL to inhibit β-lactamases, no matter their spectrum, as previously described elsewhere.Reference Payne, Cramp, Winstanley and Knowles 1 Notably, these results obtained with TZP may reflect the increased activity of piperacillin. However, the applicable comparison is between amoxicillin and ampicillin due to the overlapping activities of these agents.
Although SAM has historically been favored for its activity against Acinetobacter, its activity is no longer observed, and its usefulness is questionable in community-based infections (eg, pyelonephritis, appendicitis, cholecystitis, complicated urinary infections, and others), which often require hospitalization and more appropriate empirical therapy protocols. For community-acquired infection of mild-to-moderate severity in adults, SAM is not recommended because of high rates of resistance to this agent among community-acquired E. coli, according to the guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Reference Solomkin, Mazuski and Bradley 8 Therefore, would nosocomial use of AMC (endovenous) not be more appropriate than SAM?
Regardless, some important factors may influence this option: First, although the addition of IBLs appears to reduce the hydrolyzing effect of β-lactamase enzymes on the β-lactam ring, their activity is diminished when a high concentration of bacteria is present, cf, “inoculum effect.”Reference Lopez-Cerero, Picon and Morillo 9 These contrasting distributions may be important because respiratory tract infections imply in a high inoculum of bacteria in a compartment where penetration of antibiotics may be impaired, whereas urinary tract infections have a more moderate inoculum and β-lactams easily concentrate in the urine. Second, the presence of other mechanisms of β-lactam resistance, such as ampC β-lactamase overproduction or additional ESBLs, certainly act by reducing the activity of BL/IBLs.Reference Tamma, Girdwood and Gopaul 10 Third, our results are disturbing because the use of any BL/IBL combination is already ineffective against >10% of resistant organisms and should not be used unless hospital surveys indicate >90% susceptibility, as indicated for quinolones, for example.Reference Solomkin, Mazuski and Bradley 8
In conclusion, our results show the superior activity of TZP among the BL/IBL agents regardless of the profiles presented by the isolates. The use of SAM must be questioned (and even replaceable by AMC) due to the high resistance rates observed. Further studies are required to confirm our findings in other nosocomial populations.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: The author reports no conflicts of interest relevant to this article.





