To the Editor—Antibiotic resistance is one of the world’s most pressing public health problems. 1 Per the Centers for Disease Control and Prevention, each year in the United States, at least 2 million people become infected with resistant bacteria and at least 23,000 die as a direct result of these infections. 2 Antimicrobial resistance occurs as a natural process in which microbes evolve. 1 , 2 Subsequently, we always need new antimicrobials to sustain with newly emerging resistant pathogens. The Generating Antibiotic Incentives Now (GAIN) Act was passed on July 9, 2012, as part of the Food and Drug Administration (FDA) Safety and Innovation trying to encourage the pharmaceutical industries to return to a specialty that is in dire need of novel drugs. 3 Here, I offer an assessment how the GAIN Act addresses barriers to new antibiotic development and to identify the gaps in the Act.
The GAIN Act aims to incentivize antibiotic drug discovery worldwide in several ways. 3 It obliges the FDA to prioritize the communication and review processes for the sponsor of an eligible new antibiotic. It also grants an extra 5 years of market protection for newly qualified antimicrobials. 3 The legislation also provides 6 more months of exclusivity if the sponsor identifies a companion diagnostic test. Between July 2012 and May 2018, a total of 20 new antimicrobial agents including antibiotics, antifungals, and monoclonal antibodies that target bacteria or fungi, have been approved by the FDA compared to 6 new agents for a 6-year period prior to the GAIN Act (Table 1). However, a 2-sample Student t test comparing the mean of number of antimicrobial agents approved 6 years before and after the Act failed to show a statistically significant difference in means, with a P value of 0.06 and a 95% confidence interval of −3.84 and 0.09. Yet there is a need to consider additional incentives that stimulate the development and approval of new antimicrobials.
Table 1 List of Antimicrobial Agents Approved Before and After the GAIN Act
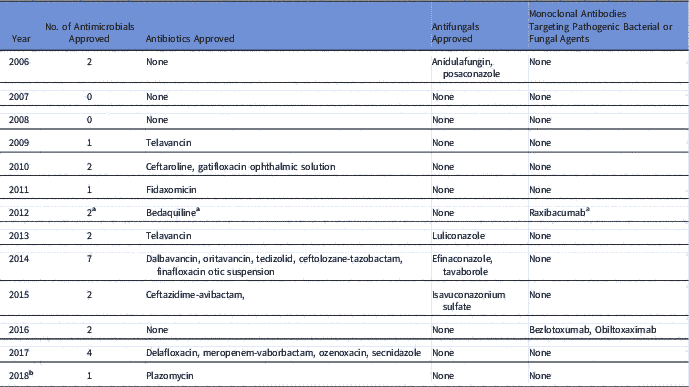
a Bedaquiline and raxibacumab were both FDA approved after July 9, 2012.
b As of May 8, 2018.
Advancements in antibiotic development are inherently challenging, particularly relative to other therapeutic fields. And the price of antibiotics is often underappreciated compared with other drugs. A main reason is that the drugs pricing in the United States is not tied to the cost-effectiveness or the public health value of the medication. Additionally, infections occur more frequently in developing countries with low insurance coverage where many patients cannot afford expensive antibacterial drugs.Reference Morse 4 , Reference Weiss and McMichael 5 Consequently, pharmaceutical industries would rather invest in developing profitable therapeutic ventures (eg, anticancer drugs, which are very expensive and yet often extend a patient’s life only by months) than in developing new antibiotics, which are often used for a short duration, which may lead to unstable demand.Reference Simpkin, Renwick, Kelly and Mossialos 6 Thus, a core incentive package (CIP) is necessary for rebalancing the antibiotics market and improving the net present value of antibiotic project development. This CIP must include both push and pull incentives.Reference Simpkin, Renwick, Kelly and Mossialos 6 , Reference Mueller-Langer 7 Push incentives focus on removing barriers to developer entry.Reference Simpkin, Renwick, Kelly and Mossialos 6 , Reference Mueller-Langer 7 That is largely accomplished by affecting the marginal cost of funds to the developer for investments in research and development by including tax credits and grants.Reference Simpkin, Renwick, Kelly and Mossialos 6 , Reference Mueller-Langer 7 These push incentives tend to impact the earlier stages of the drug development process. Meanwhile, pull incentives involve the promise of financial reward only after a technology has been developed.Reference Simpkin, Renwick, Kelly and Mossialos 6 , Reference Mueller-Langer 7 Pull incentives are largely responsible for funding and supporting the last stages of antibiotic development, including clinical trials, market approval, and commercialization.Reference Simpkin, Renwick, Kelly and Mossialos 6 , Reference Mueller-Langer 7 Large pharmaceutical industries particularly benefit from pull incentives which help guarantee a defined market for their product. Small and medium-sized enterprises can also benefit from pull incentives that pledge a future return and can help them secure venture capital to fund clinical trials.Reference Mueller-Langer 7
At present, few pull incentives are implemented in the GAIN Act including the expedited FDA review process of the new antibiotics and the extended market exclusivity. 3 However, the Act misses major pull incentives, eg, antibiotics value-based pricing (VBP) and reimbursement plans, advanced market commitments, tax incentives that subsidies firms developing antibiotics, and large market entry rewards. 3 Similarly, none of the push incentives which focus on long-term sustainable funding of basic science and preclinical research are tackled by the Act. 3 Hence, incorporating into the Act the comprehensive CIP would create a robust competition among manufacturers of antimicrobials and generics and result in price competition.
Furthermore, drug pricing is always a complicated process. For pharmaceutical industries, it is important to make profit on the drugs sales because of the large investments needed to develop a new drug. However, due to the limited budget, and the rising proportion of health spending that is accounted for by pharmaceutical industries, it is important to investigate whether the drugs provide value for money. Value-based pricing is a method that could be used to determine a price for new antibacterial agents at which these drugs provide value to the patient and health system rather than per the cost of the drug or historical prices.Reference Verhoef and Morris 8 , Reference Robinson, Howell and Pearson 9 This VBP reflects an assessment of the comparative effectiveness of the drug compared to other available treatments. An important factor will be the definition of “value,” which might also include other factors than the impact of the drug on patient health, that is, wider social impact and the health impact of disease.Reference Verhoef and Morris 8
In conclusion, although the GAIN Act has been a laudable initiative aiming to reduce the devastating burden of antimicrobial resistance, substantial barriers remain. Accordingly, there is a need for new policies that incorporate the CIP to incentivize antibiotics innovation and create better solutions for the devastating burden of antimicrobial resistance. Equally important, VBP of the new antibiotics is essential throughout the innovative process.
Acknowledgments
None to disclose.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
The author reports no conflicts of interest relevant to this article.





