Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by Crossref.
Madden, Gregory R.
German Mesner, Ian
Cox, Heather L.
Mathers, Amy J.
Lyman, Jason A.
Sifri, Costi D.
and
Enfield, Kyle B.
2018.
Reduced Clostridium difficile Tests and Laboratory-Identified Events With a Computerized Clinical Decision Support Tool and Financial Incentive.
Infection Control & Hospital Epidemiology,
Vol. 39,
Issue. 6,
p.
737.
Madden, Gregory R.
Poulter, Melinda D.
and
Sifri, Costi D.
2018.
Diagnostic stewardship and the 2017 update of the IDSA-SHEA Clinical Practice Guidelines for Clostridium difficile Infection.
Diagnosis,
Vol. 5,
Issue. 3,
p.
119.
Emberger, Jennifer
Tassone, Dan
Stevens, Michael P.
and
Markley, J. Daniel
2018.
The Current State of Antimicrobial Stewardship: Challenges, Successes, and Future Directions.
Current Infectious Disease Reports,
Vol. 20,
Issue. 9,
O'Connor, Ciara
McGuinness, Claire
Cafferty, Deirdre
Cunney, Robert
and
Drew, Richard J.
2019.
Diagnostic stewardship in the post-vaccine era: Reducing demand for meningococcal and pneumococcal PCR.
Journal of Infection,
Vol. 78,
Issue. 1,
p.
75.
Lin, Grace
Knowlson, Shelley
Nguyen, Huong
Cooper, Kaila
Pryor, Rachel J.
Doll, Michelle
Godbout, Emily J.
Hemphill, Robin
Stevens, Michael P.
and
Bearman, Gonzalo
2019.
Urine test stewardship for catheterized patients in the critical care setting: Provider perceptions and impact of electronic order set interventions.
American Journal of Infection Control,
Vol. 47,
Issue. 10,
p.
1277.
Civitarese, Anna M.
Ruggieri, Eric
Walz, J. Mattias
Mack, Deborah Ann
Heard, Stephen O.
Mitchell, Michael
Lilly, Craig M.
Landry, Karen E.
and
Ellison, Richard T.
2019.
RE: Preventability of hospital onset bacterermia and fungemia: A pilot study of potential healthcare-associated infection outcome measure, by Dantes et al (2019).
Infection Control & Hospital Epidemiology,
Vol. 40,
Issue. 10,
p.
1209.
Fleming, Michele S.
Hess, Olivia
Albert, Heather L.
Styslinger, Emily
Doll, Michelle
Nguyen, Huong Jane
McAulay-Kidd, Susan
Hemphill, Robin R.
Srivastava, Tara
Cooper, Kaila D.
Stevens, Michael P.
and
Bearman, Gonzalo
2019.
Test stewardship, frequency and fidelity: Impact on reported hospital-onset Clostridioides difficile.
Infection Control & Hospital Epidemiology,
Vol. 40,
Issue. 6,
p.
710.
Muniz de Oliveira, Rafael
da Rosa Gioppo, Nereida Mello
Oliveira de Carvalho, Jancineide
Carvalho Oliveira, Francilio
Webster, Thomas Jay
Marciano, Fernanda Roberta
and
Oliveira Lobo, Anderson
2019.
Decontamination of mobile phones and electronic devices for health care professionals using a chlorhexidine/carbomer 940® gel.
Frontiers of Chemical Science and Engineering,
Vol. 13,
Issue. 1,
p.
192.
Tung, William
and
Hays, Rachel
2019.
Atypical presentation ofClostridioides difficilepseudomembranous colitis with laboratory rejection of stool specimen.
BMJ Case Reports,
Vol. 12,
Issue. 11,
p.
e230629.
Bearman, Gonzalo
Doll, Michelle
Cooper, Kaila
and
Stevens, Michael P.
2019.
Hospital Infection Prevention: How Much Can We Prevent and How Hard Should We Try?.
Current Infectious Disease Reports,
Vol. 21,
Issue. 1,
Doll, Michelle
Fleming, Michele
Stevens, Michael P.
and
Bearman, Gonzalo
2019.
Clostridioides difficile–Associated Diarrhea: Infection Prevention Unknowns and Evolving Risk Reduction Strategies.
Current Infectious Disease Reports,
Vol. 21,
Issue. 1,
Madden, Gregory R.
Cox, Heather L.
Poulter, Melinda D.
Lyman, Jason A.
Enfield, Kyle B.
and
Sifri, Costi D.
2019.
Cost Analysis of Computerized Clinical Decision Support and Trainee Financial Incentive for Clostridioides difficile Testing.
Infection Control & Hospital Epidemiology,
Vol. 40,
Issue. 2,
p.
242.
Meninger, Susanne
Fadlallah, Hasan
Dowler, Karen
and
Doron, Shira
2020.
Quality Measures.
p.
47.
Nkemngong, Carine A.
Voorn, Maxwell G.
Li, Xiaobao
Teska, Peter J.
and
Oliver, Haley F.
2020.
A rapid model for developing dry surface biofilms of Staphylococcus aureus and Pseudomonas aeruginosa for in vitro disinfectant efficacy testing.
Antimicrobial Resistance & Infection Control,
Vol. 9,
Issue. 1,
Appaneal, Haley J.
Caffrey, Aisling R.
Hughes, Maria-Stephanie A.
Lopes, Vrishali V.
Jump, Robin L.P.
LaPlante, Kerry L.
and
Dosa, David M.
2020.
Trends in Collection of Microbiological Cultures Across Veterans Affairs Community Living Centers in the United States Over 8 Years.
Journal of the American Medical Directors Association,
Vol. 21,
Issue. 1,
p.
115.
Dbeibo, Lana
Brinkman, Allison
Beeler, Cole
Fadel, William
Snyderman, William
Hatfield, Nicole
Sadowski, Joshua
Wang, Yun
Kelley, Kristen
Webb, Douglas
Azar, Jose
and
Kara, Areeba
2020.
Utilizing a real-time discussion approach to improve the appropriateness of Clostridioides difficile testing and the potential unintended consequences of this strategy.
Infection Control & Hospital Epidemiology,
Vol. 41,
Issue. 10,
p.
1215.
Savoldi, Alessia
Gentilotti, Elisa
De Nardo, Pasquale
Razzaboni, Elisa
Bovo, Chiara
and
Carrara, Elena
2020.
Ushering in Diagnostic Stewardship: a Step Towards Antibiotic Stewardship.
Current Treatment Options in Infectious Diseases,
Vol. 12,
Issue. 3,
p.
202.
Madden, Gregory R
Enfield, Kyle B
and
Sifri, Costi D
2020.
Patient Outcomes With Prevented vs Negative Clostridioides difficile Tests Using a Computerized Clinical Decision Support Tool.
Open Forum Infectious Diseases,
Vol. 7,
Issue. 4,
Morjaria, Sejal
and
Chapin, Kimberle C.
2020.
Who to Test, When, and for What.
The Journal of Molecular Diagnostics,
Vol. 22,
Issue. 9,
p.
1109.
Church, Deirdre L.
and
Naugler, Christopher
2020.
Essential role of laboratory physicians in transformation of laboratory practice and management to a value-based patient-centric model.
Critical Reviews in Clinical Laboratory Sciences,
Vol. 57,
Issue. 5,
p.
323.





Healthcare-associated infections (HAIs) are associated with increased morbidity and mortality, prolonged hospital stays, and unnecessary cost. The financial stakes of HAIs for hospitals were underscored in 2008 when the Centers for Medicare and Medicaid Services (CMS) began to withhold payment for certain “reasonably preventable” HAIs, including catheter-associated urinary tract infections (CAUTIs), central line-associated bloodstream infections (CLABSIs), and surgical site infections (SSIs). 1
Most current efforts to reduce HAIs focus on strategies to prevent infection without addressing unnecessary testing or diagnostic error; however, a false-positive test result that provides an erroneous diagnosis of an HAI may lead to increased cost and possible harm to the patient, although data quantifying these effects are lacking. Accurate diagnostics are critical for safe patient care and have additional impacts in our environment of value-based payment, public reporting, and quality metrics, where hospitals may incur penalties for HAI test overuse, including lost reimbursement, financial penalties, and damage to institutional reputation and rankings. From a patient care perspective, overdiagnosis of HAIs could lead to inappropriate antimicrobial use and attendant unnecessary cost and risks antimicrobial resistance and adverse drug effects.
DIAGNOSTIC STEWARDSHIP CONCEPT AND ROLE IN HAIs
Developed more than 20 years ago, antimicrobial stewardship programs (ASPs) can play a key role in reducing cost, antimicrobial resistance, and some HAIs. Studies suggest that ASPs are most effective when coupled with infection prevention strategies.Reference Lawes, Lopez-Lozano and Nebot 2 Overall, ASPs are widely adopted and regarded as safe and have not been found to increase patient mortality or other patient-centered adverse outcomes, despite reduced antimicrobial use.Reference Kaki, Elligsen, Walker, Simor, Palmay and Daneman 3 Recognizing this, The Joint Commission now requires ASPs for hospital accreditation, and the CMS has proposed ASP standards in acute-care hospitals, critical-access hospitals, and long-term care facilities. 4
Diagnostic stewardship practices are increasingly common among hospitals, often classified as quality improvement or under the umbrella of antimicrobial stewardship. Examples include targeted staff education with regard to test ordering, interpretation, or proper specimen collection, as well as laboratory “prior authorization” policies designed to limit tests. In the near future, the CMS may begin to require diagnostic stewardship in the form of an approved clinical-decision support system, to receive full payment for advanced diagnostic imaging tests (through the Appropriate Use Criteria program established under the Protecting Access to Medicare Act of 2014, pending final approval by the CMS). 5
Diagnostic stewardship has a potentially important role in HAI surveillance. The Centers for Disease Control and Prevention (CDC), through the National Healthcare Safety Network (NHSN), monitors >70% of all US hospitals for several hospital-related infections including SSI, CLABSI, CAUTI, ventilator-associated pneumonia (VAP) (now more broadly characterized as a possible ventilator-associated pneumonia, or PVAP), and healthcare-facility-onset Clostridium difficile infection (HO CDI). 4 Surveillance-based definitions, such as those developed by the NHSN for HAI events, are pragmatically designed for surveillance purposes and are not intended for use in the clinical evaluation and care of patients. For example, current NHSN surveillance definitions for HO CDI require only a positive test for C. difficile from an unformed stool specimen on or after hospital day 4, irrespective of patient symptoms, clinical condition, alternative diagnoses, or multistep testing laboratory algorithms, whereas clinical practice guidelines require clinical indications of disease and advocate that testing of asymptomatic patients is not clinically useful. 6 , Reference Cohen, Gerding and Johnson 7 Many surveillance definitions cannot necessarily be used to distinguish true infections from false-positive tests.
Overuse of tests is predicted to increase false positives that trigger needless downstream cost and treatment that may cause harm for the patient. Conversely, test underuse risks missed diagnoses and potential harm related to untreated conditions. As with antimicrobial utilization, we hypothesize that there exists a state of optimal test use for HAIs in at-risk patients.
HAI rates based on surveillance definitions may over-diagnose CAUTI, CLABSI, HO CDI, hospital-acquired pneumonia (HAP), and VAP, estimated up to 37%,Reference Trautner, Cope, Cevallos, Cadle, Darouiche and Musher 8 30%,Reference Boyce, Nadeau and Dumigan 9 15%–53%,Reference Kelly, Yarrington and Zembower 10 , Reference Polage, Gyorke and Kennedy 11 47%,Reference Burton, Price and Barr 12 and 58%–68%,Reference Nussenblatt, Avdic and Berenholtz 13 , Reference Klompas, Kulldorff and Platt 14 respectively. Furthermore, the results of new, highly sensitive molecular diagnostics that detect minute amounts of a microbial target, such as nucleic acid amplification testing (NAAT) for C. difficile toxin gene(s), may identify colonized rather than clinically infected patients. This misattribution of colonized patients can artificially increase HAI rates.Reference Polage, Gyorke and Kennedy 11
Diagnostic stewardship is defined as coordinated systems or user-based interventions designed to promote evidence-based utilization of diagnostic tests, with the primary goals of improving value and care quality and safely reducing cost. It has the potential to address falsely inflated HAI rates due to overtesting.Reference Morgan, Malani and Diekema 15 , Reference Durand, Lewin and Berkowitz 16 Diagnostic stewardship has been described recently by Morgan et alReference Morgan, Malani and Diekema 15 to occur in three stages: preanalytic (test-related decision making and specimen collection), analytic (relating to laboratory practices including protocolized or reflex test algorithms), and postanalytic (eg, selective reporting of antimicrobial susceptibility data to encourage the use of narrower spectrum agents).
Diagnostic stewardship has been shown to effectively reduce a variety of unnecessary general inpatient medicine tests, from excessive or redundant daily inpatient labs to diagnostic imaging.Reference Durand, Lewin and Berkowitz 16 , Reference McDonald, Saleh and Lee 17 Diagnostic stewardship strategies are varied and include user-based approaches (eg, auditing, price display, and provider feedback) and systems-based approaches (eg, modifications to the computerized physician order entry (CPOE) system requiring selection of an indication for testing and inappropriate specimen rejection).
IMPLEMENTATION CHALLENGES AND SAFETY CONCERNS
While reducing unnecessary tests for HAIs can have many potential benefits for the patient and hospital, test underutilization raises the possibility for serious infections going undiagnosed and untreated. For example, while excessive C. difficile testing may identify patients with colonization or resolving infections (which is not only a waste of resources but also leads to unnecessary treatment), more restricted testing might result in unrecognized and untreated CDI (resulting in harm to individual patients and greater risk of cross infection) or empiric treatment for CDI without testing as a workaround (resulting in unnecessary treatment in a subset of patients). A major objective for diagnostic stewardship for HAIs is to identify the “sweet spot” of test utilization that minimizes overdiagnosis and false positive results while maximizing appropriately indicated testing and true positive results. This spot likely will be infection and population (eg, disease prevalence) specific.
Because HAI-related tests pose unique risks associated with reduced testing, which outcomes should be tracked to monitor patient safety? General outcome measures, as in ASPs, could include length of stay, antimicrobial resistance rates, antimicrobial use, CDI rates, mortality, and readmission. Potential comorbid complications tailored to the HAI(s) in question are also an essential stopgap that should prompt reconsideration for testing. For instance, following the introduction of a “stewardship of culturing” aimed at reducing CAUTIs, Mullin et alReference Mullin, Kovacs and Fatica 18 monitored overall rates of hospital-acquired (HABSI) infections, given the potential for complications of untreated urinary tract infection. However, outcome data in this and other HAI-related diagnostic stewardship studies were collected in aggregate and were not stratified to patients for whom the test was prevented and thus were at the highest risk for untreated infection. Ideally, prospective monitoring for HAIs should be performed for patients before and after diagnostic stewardship interventions to assess the direct patient-centered impact of these interventions in addition to aggregate data. These safety measures have largely been overlooked in the limited literature to date that has assessed diagnostic stewardship for HAIs, and incorporation in future studies presents significant logistical hurdles. Discordance between surveillance and clinical definitions for HAIs or those without a clear gold-standard clinical definition (eg, CDI) present challenges to evaluating safety when differentiating true positives remains elusive.
Similar to ASPs, there is no one-size-fits-all approach to reducing unnecessary HAI tests among all institutions. Information technology and CPOE capabilities, population characteristics, local ordering practices, HAI incidence, and laboratory test performance characteristics should all be taken into account when developing a diagnostic stewardship approach. Institutional factors, such as laboratory and stewardship activity, hospital administration support, and barriers such as provider pushback, are additional factors to consider. As with any quality improvement effort, process measures are also vital to ensure that stewardship interventions are having their intended effects, such as testing rates (including tests that are rejected from processing) and rates of the target HAI.
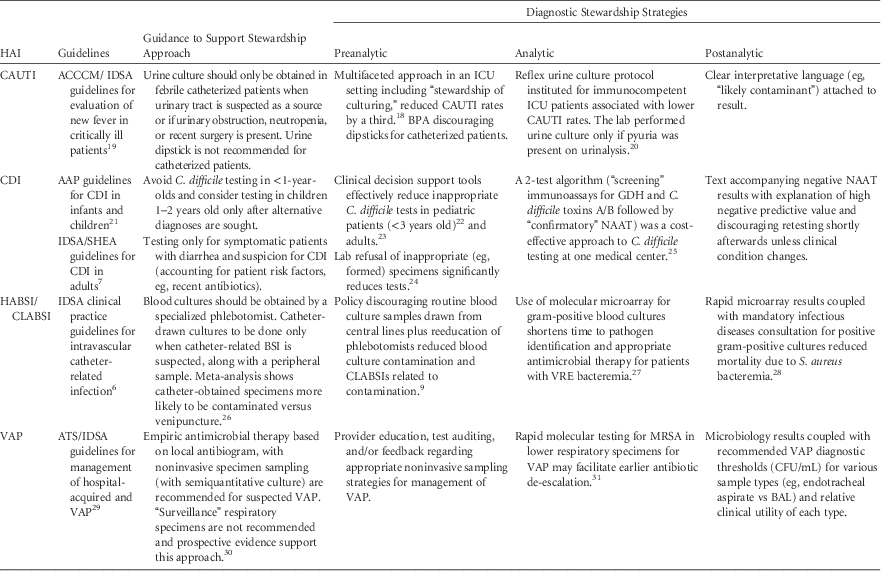
Table 1, incorporating the stages-of-testing concept of Morgan et al,Reference Morgan, Malani and Diekema 15 lists examples of diagnostic stewardship strategies for HAIs from the literature as well as other potential strategies that could be used to optimize test utilization. As in ASPs, engineered flexibility is key in the event that special circumstances require deviation from prescribed practices, the diagnostic stewardship strategy fails to achieve intended goals, or patient harm is detected.
TABLE 1 Examples of HAI-related Diagnostic Stewardship Strategies
NOTE. CAUTI, catheter-associated urinary tract infection; BPA, best practice alert; ACCCM, American College of Critical Care Medicine; CDI, Clostridium difficile infection; AAP, American Academy of Pediatrics; IDSA, Infectious Diseases Society of America; SHEA, Society for Healthcare Epidemiology of America; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification test; HABSI, hospital-acquired bloodstream infection; CLABSI, central-line associated bloodstream infection; VRE, vancomycin-resistant Enterococcus; VAP, ventilator-associated pneumonia; ATS, American Thoracic Society; MRSA, methicillin-resistant Staphylococcus aureus; CFU, colony-forming units; BAL, bronchoalveolar lavage.
CONCLUSIONS
Clinicians are faced with increasingly complex medical problems and varying test sensitivity and specificity that usually are not apparent to those ordering tests. Thus, understanding how to limit false positives without restricting appropriate testing has become a major challenge as well as an important opportunity for improving hospital infection control, infection prevention, and patient safety. As new diagnostic technologies proliferate, key metrics like clinical relevance and cost-effectiveness must be considered before such technologies are incorporated into clinical practice, and systems must be in place for stewardship of each new test before it is introduced into clinical practice.Reference Messacar, Parker, Todd and Dominguez 32
Established testing recommendations (preferably from professional societies or governing medical bodies) are essential to developing a stewardship strategy; however specific, useful consensus guidelines for diagnostic testing for HAIs are often lacking. For instance, no clear consensus exists to guide the use of repeated blood culturing to minimize false-positive rates and maximize true positives, as in patients with repeated fevers and/or patients who are already on antibiotics.Reference Linsenmeyer, Gupta, Strymish, Dhanani, Brecher and Breu 33
Developing meaningful guidelines for diagnostic stewardship for HAIs requires quality evidence from thoughtfully conducted clinical studies. Much work remains to be done to determine the safety and efficacy of limiting providers’ autonomy for HAI-related diagnostics. Outcomes and safety-oriented quality improvement research may help bridge the gap between clinical research and practice.
A combined diagnostic and antimicrobial stewardship model could promote better patient evaluations, test choices, interpretations of results, and decisions to prescribe antimicrobial therapy.Reference Messacar, Parker, Todd and Dominguez 32 Expanding on the success of antimicrobial stewardship, diagnostic stewardship should take a multidisciplinary, collaborative approach to existing best practices for HAI prevention.
ACKNOWLEDGMENTS
Financial support: The study was supported by the National Institutes of Health Infectious Diseases Training Grant (no. 5T-32AI007046-41).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.