The introduction of antimicrobials has transformed medical practice converting previously fatal infections into treatable diseases. Misuse and overuse of antimicrobials comprise a significant cause of emerging antimicrobial resistance (AMR). 1 Although early and appropriate treatment has been shown to reduce mortality Reference Dellinger, Levy and Rhodes2 in patients with severe sepsis or septic shock, 20%–50% of antimicrobials prescribed in US hospitals are inappropriate or unnecessary. Reference Camins, King and Wells3–Reference Levin, Idrees, Sprung, Weissman, Weiss, Moses and Benenson5 Furthermore, antimicrobial exposure increases the risk of adverse events, drug interactions, superinfections, and the development of multidrug-resistant organisms (MDROs), fungal infections, Clostridiodes difficile infection (CDI), as well as healthcare costs. Reference Alshammari, Larrat, Morrill, Caffrey, Quilliam and Laplante6–Reference Lapi, Wilchesky, Kezouh, Benisty, Ernst and Suissa9 Inappropriate use of antimicrobials has a negative impact not only on the patient but also on the broader patient population through increased rates of MDROs. Reference Huttner, Harbarth and Carlet10
Estimates indicate that >700,000 deaths occur worldwide each year from AMR, and this number is projected to reach 10 million in 2050. Reference O’Neill11 The financial costs associated with treating these infections reach many billions of dollars. 1,Reference Davies, Grant and Catchpole12 The effective implementation of antimicrobial stewardship programs (ASPs) has allowed a cost-effective reduction in antimicrobial consumption, increasing patient safety and reducing AMR. Reference Davey, Brown and Charani13,Reference Malani, Richards, Kapila, Otto, Czerwinski and Singal14 The implementation of strategies for appropriate use of antimicrobials is a cornerstone in reducing emergence and transmission of MDROs. Multiple guidelines have been published, proposing a framework to combat AMR based on the development of robust infection control programs, therapeutic committees, guidelines on antimicrobial management, and monitoring and feedback of prescription patterns. Reference Dellit, Owens and McGowan15–Reference Barlam, Cosgrove and Abbo18 This framework contributed to the development of ASPs, which focus on preprescription authorization and postprescription review and feedback, which have succeeded at curbing resistance, decreasing costs, and decreasing rates of CDI in US hospitals. 19–Reference Elligsen, Walker and Pinto21 However, hospitals located in low- and middle-income countries (LMICs) have different sets of challenges. Reference Bebell and Muiru22,Reference Dapás and Quirós23 Stories of modest success have been reported around the world, but robust data are lacking, Reference Quirós24 and data on AMR and ASPs in Latin America are especially scarce.
In this study, we aimed to implement ASPs in adult medical-surgical intensive care units (MS-ICUs) from Latin American countries and to assess impact on appropriateness of antimicrobial prescriptions, antimicrobial use, crude mortality, MDRO in healthcare-associated infections (MDRO-HAIs) and Clostridioides difficile infections (CDIs). We hypothesized that MS-ICUs with higher scores in the final self-assessment would show improved indicators of appropriate antimicrobial use and improved patient outcomes.
Methods
Study design
The study included a network of hospitals recruited from 9 Latin American countries. A nonrandom sample of 84 MS-ICUs from tertiary-care hospitals in Latin America were invited by infectious disease leaders from each country to voluntarily participate in the project. We included facilities with an ASP team composed of an infectious disease (ID) physician, a clinical pharmacist, and a microbiologist. All study data were deidentified, and patient consent was waived. Ethics approvals varied by country and were obtained by participating hospitals on an individual basis. A central ethics committee was enlisted at the coordinating center, the Global Health Initiative at Henry Ford Health System, Michigan. A data privacy document was made available for each participating hospital.
The study was built using methodology from a prior study. Reference Quirós, Cabral and Bertuzzi25 The study was conducted over a 24-month period with a 6-month preintervention period, a 12-month intervention period, and a 6-month postintervention study period.
During the preintervention period, the members of the ASP teams were trained through an online course. During this period, each center completed a baseline self-assessment of their ASP through a previously validated instrument (Supplementary Material 1 online). Reference Quirós, Cabral and Bertuzzi25
During the 12-month intervention period and based on the results of the baseline self-assessment, each center implemented locally salient antimicrobial stewardship strategies in MS-ICUs as part of the ASP. During this period, monthly surveys were performed to measure the appropriateness of antimicrobial prescription, antimicrobial consumption, mortality, and incidence of MDRO-HAIs. The implementations of respective IPC strategies were registered monthly. At month 6 during the intervention period, an interim self-assessment using the same instrument was performed to respectively determine which ASP strategies had been implemented.
During the postintervention period, a final self-assessment was performed to summarize the level of development achieved by the ASP at each MS-ICU.
Data collection
All data were entered into a secured online database through a central website developed for this study. A help desk and supplementary documents were available there. The platform included the online training course, participating center characteristics, and standardized collection of study information. Data validation included several integrated verifications with error and warning messages to avoid duplicated and erroneous data entry and missing information. The system created monthly indicators and graphics comparing data from all participating MS-ICUs. Each investigator could monitor their own indicators in comparison with the rest of centers.
The following variables were collected from each participating hospital for analysis and comparison: affiliation type (public or private, teaching or nonteaching), number of MS-ICU beds, full-time equivalent for ASP team members, and the presence of an IPC committee and/or a pharmacy committee.
The instrument used to evaluate the ASPs was based on the CDC Core Elements checklist 26 and contained a total of 74 indicators, grouped into 33 standards, 15 components, and 4 domains (Supplementary Material 1 online). We evaluated the following domains: (1) leadership and coordination, (2) institutional intervention strategies, (3) monitoring, and (4) education. Partial scores for each domain and a global score were developed. The results of the self-assessment of ASPs were finalized on a scale from 0 to 100 points to allow institutional comparisons. The means of the self-assessment score were grouped by percentiles (≤25th, >25th to <75th, and ≥75th) for comparisons among the MS-ICUs.
A standardized surveillance methodology was used to collect data from antimicrobial prescriptions. Reference Quirós, Cabral and Bertuzzi25,Reference Versporten, Zarb and Caniaux27 Monthly 1-day prevalence surveys, including all inpatients who were in an MS-ICU at 8:00 a.m. and who received at least 1 systemic antimicrobial, were collected. Survey information included characteristics of the patient (ie, age, gender, weight) and the antimicrobial prescription (ie, therapeutic indication, unit dose, number of daily doses, administration route, pharmacist validation, dose adjustments, and therapeutic drug monitoring). Prescriptions were categorized as treatment for community-acquired infections (CAIs), treatment for healthcare-associated infections (HAIs), medical prophylaxis, or surgical prophylaxis. Whether treatment was empirical or targeted was also recorded.
We analyzed the following indicators of appropriateness of the antimicrobial prescriptions: surgical prophylaxis <24 hours, validation of prescription by pharmacists, justification of prescription in the medical record, compliance with clinical guidelines, prospective audit and feedback, acceptance of ID physician recommendation, aminoglycosides in a 1-day dose, no redundant anaerobic therapy, de-escalation of therapy performed, and switch from intravenous to oral route.
Antimicrobial consumption was measured in defined daily doses per 100 patient days (DDD per 100 PD) per month for systemic antibacterials and antifungals (categories J01 and J02 from the Anatomical Therapeutic Chemical classification system). 28,Reference Polk, Fox, Mahoney, Letcavage and MacDougall29
To determine the impacts of the respective ASPs, monthly CDI, MDRO-HAIs, and all-cause mortality in the MS-ICU were registered. Additionally, the respective implementations of the following IPC strategies were registered: hand hygiene program, periodic surveillance of MDRO-HAIs, policies for contact precautions and environmental cleaning, daily chlorhexidine bathing, surveillance, and bundles addressing device-associated infections.
Statistical analysis
The results of the self-assessment are shown as mean ± SD. The paired Student t test was used to compare the initial and final scores, and the Student t test was used to compare MS-ICUs. To identify institutional characteristics associated with the level of the ASP, a bivariate analysis was conducted using the final self-assessment score as the outcome. Statistically associated variables (P < .10) were introduced into a stepwise multiple linear regression model, and only those that were significantly associated (P < .05) remained in the model.
Indicators for appropriateness of antimicrobial prescriptions were presented as percentage of total prescriptions complying with each indicator. Overall, crude mortality was expressed as monthly death in the MS-ICU per 100 discharges. Cumulative compliance of IPC strategies was calculated as percentage of the 12 months each strategy was implemented. These variables were compared using the χ Reference Dellinger, Levy and Rhodes2 statistic, and results are expressed as differences of the percentages and their respective 95% CIs.
The total DDD per 100 PD of targeted antimicrobials, MDRO-HAIs, and CDIs at the different MS-ICUs were compared as incidence densities using the Poisson test.
A P value <.05 (2-tailed) was considered statistically significant. For statistical analyses, we used SPSS version 22 software (IBM, Chicago, IL).
Results
Of the 84 MS-ICUs that initially agreed to participate in the project, 77 (91.7%) completed the study and 7 (8.3%) dropped out. Of the participating sites, 45 were from Argentina, 6 were from Ecuador, 5 were from Colombia, 5 were from Uruguay, 5 were from Brazil, 3 were from Chile, 3 were from Peru, 3 were from Panama, and 2 were from Bolivia. Overall, 233 members of ASP teams completed the online training course.
Self-assessment
The global scores of the initial and final self-assessments were 40.7 ± 17.3 and 52.1 ± 19.2, respectively (difference, 11.3; 95% CI, 8.1 to 14.6; P < .0001), and all 4 domains showed a significant improvement over the course of the study (Table 1).
Table 1. Comparison at the Domains Between Initial and Final Self-Assessment
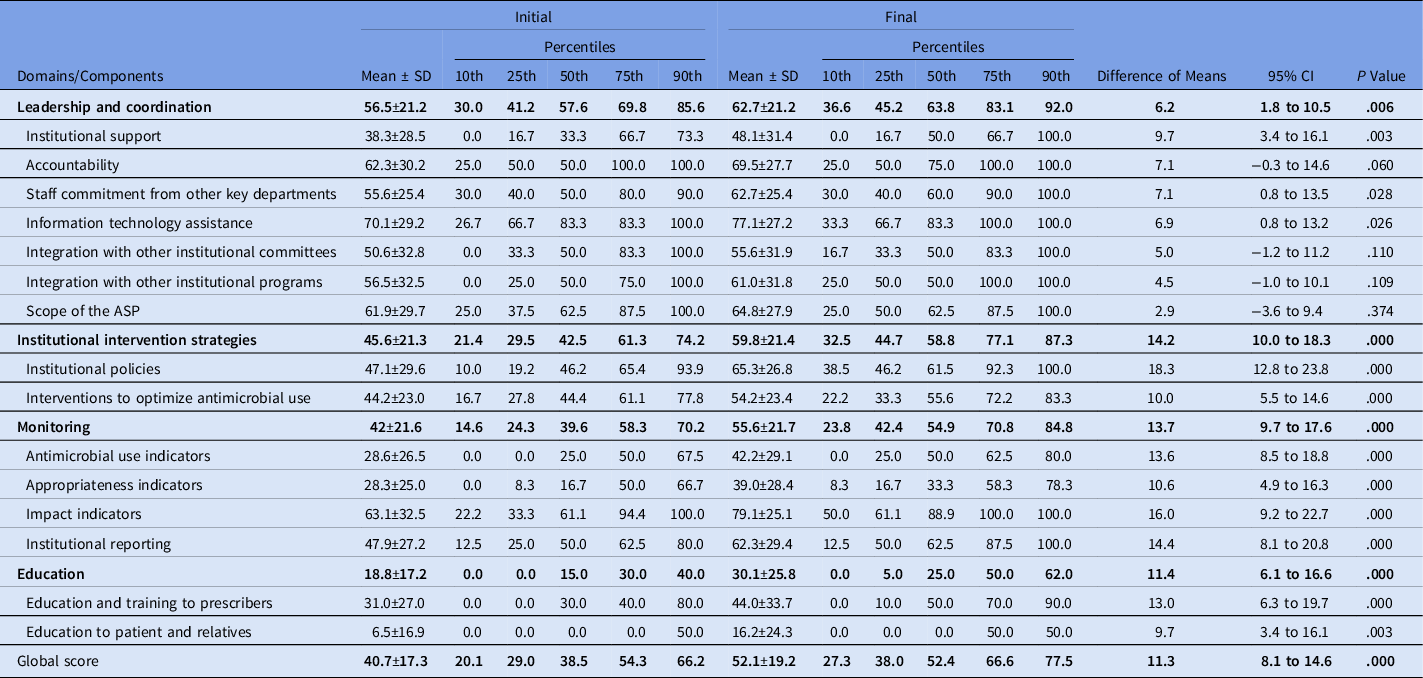
Note. SD, standard deviation; CI, confidence interval; ASP, antimicrobial stewardship program.
The following components showed significant improvement in the final self-assessments: institutional support, staff commitment from other key departments, information technology assistance, institutional policies, interventions to optimize antimicrobial use, monitoring of antimicrobial use, appropriateness, impact indicators, and education and training for prescribers and for patients and relatives (Table 1).
Only Argentina, Colombia, Panama, and Uruguay showed a significant improvement in the global scores per country when the initial and final self-assessments were compared (Table 2).
Table 2. Comparison of Global Scores per Country Between Initial and Final Self-Assessment
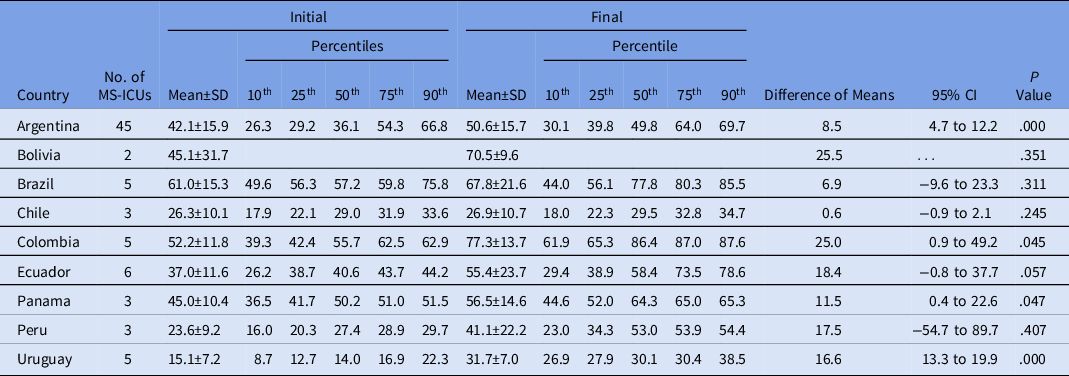
Note. MS-ICU, medical-surgical intensive care unit; SD, standard deviation; CI, confidence interval.
The following institutional characteristics associated with a higher global score in the final self-assessment in the bivariate analysis: private versus public institution (56.2 vs 47.6; P = .048); at least 15 beds in the MS-ICU (58.7 vs 48.5; P = .024); full-time infection preventionist (53.1 vs 25.9; P = .015); at least 6 meetings per year of the IPC committee (55.4 vs 43.1; P = .011); and at least 6 meetings per year of the pharmacy committee (58.9 vs 47.4; P = .009). In the stepwise multiple linear regression, only facilities with at least 15 beds at the MS-ICU and at least 6 meetings per year of the pharmacy committee showed an independent significant statistical association (P = .042 and P = .015, respectively).
When institutions were stratified into 3 groups according to the global score of the final self-assessment (ie, ≤25th,>25th to <75th, ≥75th percentiles), only those centers in the >25th to <75th percentile and ≥75th percentile groups showed a significant improvement in their ASPs when the final and initial self-assessments were compared (52.1 vs 38.1 and 76.1 vs 59.2, respectively; both P < .0001). Centers within the 25th percentile did not show significant improvement in their ASPs (28.0 vs 27.1; P = .7077).
Antimicrobial prescriptions, antimicrobial consumption, and appropriateness indicators
Data from the point-prevalence surveys showed that of 10,058 MS-ICU inpatients, 6,019 inpatients (59.8%) received 10,523 antimicrobial prescriptions (1.75 antimicrobial per patient on antimicrobial treatment). Of these 10,523 prescriptions, 5,194 (49.4%) corresponded to HAIs treatment; 2,992 (28.4%) for CAIs; 590 (5.6%) surgical prophylaxis; 458 (4.4%) for medical prophylaxis; and 1,289 (12.2%) for indications not classified. Targeted treatments were significantly more common for HAIs than for CAIs (43.9% vs 25.1%; P < .0001).
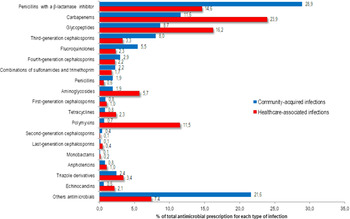
Among all antibiotics prescribed for CAIs, the following were most frequently prescribed: 663 prescriptions (28.9%) were for penicillins with a β-lactamase inhibitor, 202 (8.8%) were for meropenem, 199 (8.7%) were for vancomycin, and 184 (8.0%) were for a third-generation cephalosporin (Fig. 1). Among the prescriptions for penicillins with a β-lactamase inhibitor, 314 prescriptions (13.7%) were for piperacillin with a β-lactamase inhibitor and 312 (13.6%) were for amoxicillin with a β-lactamase inhibitor. The following antibiotics were most commonly prescribed for HAIs: 1,242 prescriptions (23.9%) were for carbapenems, 840 (16.2%) were for glycopeptides, 758 (14.6%) were for penicillins with a β-lactamase inhibitor, and 597 (11.5%) were for polymyxins (Fig. 1).
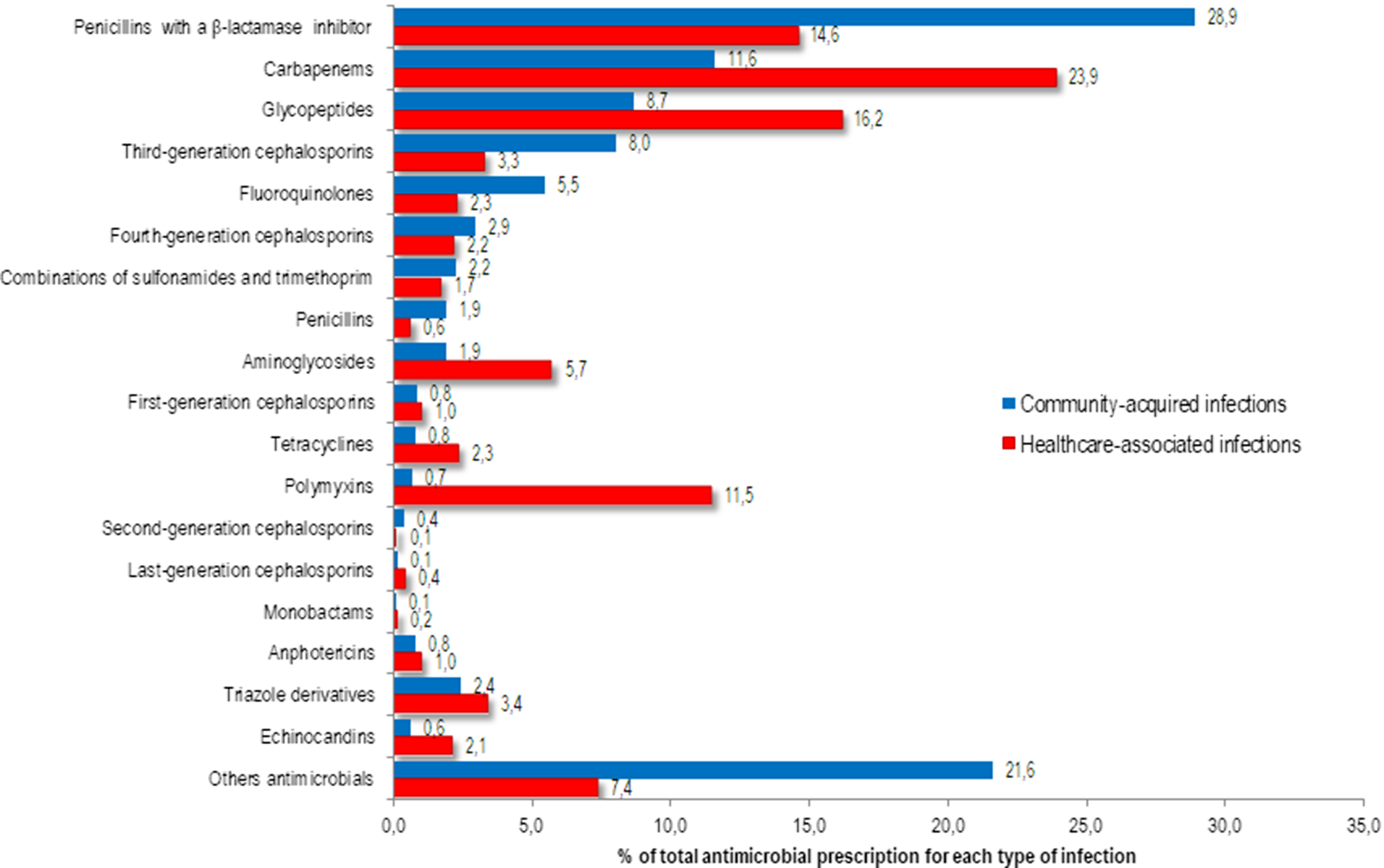
Fig. 1. Proportion of antimicrobials prescribed for systemic use in community-acquired (n=2292) and healthcare-associated (n=5194) infections among adult patients in medical-surgical ICU.
Among the 426 systemic antifungal prescriptions, triazole drugs were the most frequently prescribed: 55 of 87 CAIs (2.4% of total prescriptions) and 177 of 339 HAIs (3.4% of total prescriptions) (Fig. 1).
Throughout the 12-month study period, 464,770 DDDs were consumed throughout 304,700 patient days in MS-ICUs (152.5 DDD per 100 PD). The following antimicrobial groups were most frequently consumed: penicillins with a β-lactamase inhibitor (34.0 DDD per 100 PD), followed by carbapemens (30.4 DDD per 100 PD), glycopeptides (13.2 DDD per 100 PD), and polymyxins (9.7 DDD per 100 PD).
The frequency distribution of antimicrobial consumption between MS-ICUs stratified by the final self-assessment into the 3 percentile groups are shown in Figure 2. Consumption of penicillin with a β-lactamase inhibitor, carbapenem, glycopeptide, third-generation cephalosporin, and aminoglycoside were lower in MS-ICUs in the ≥75th percentile.
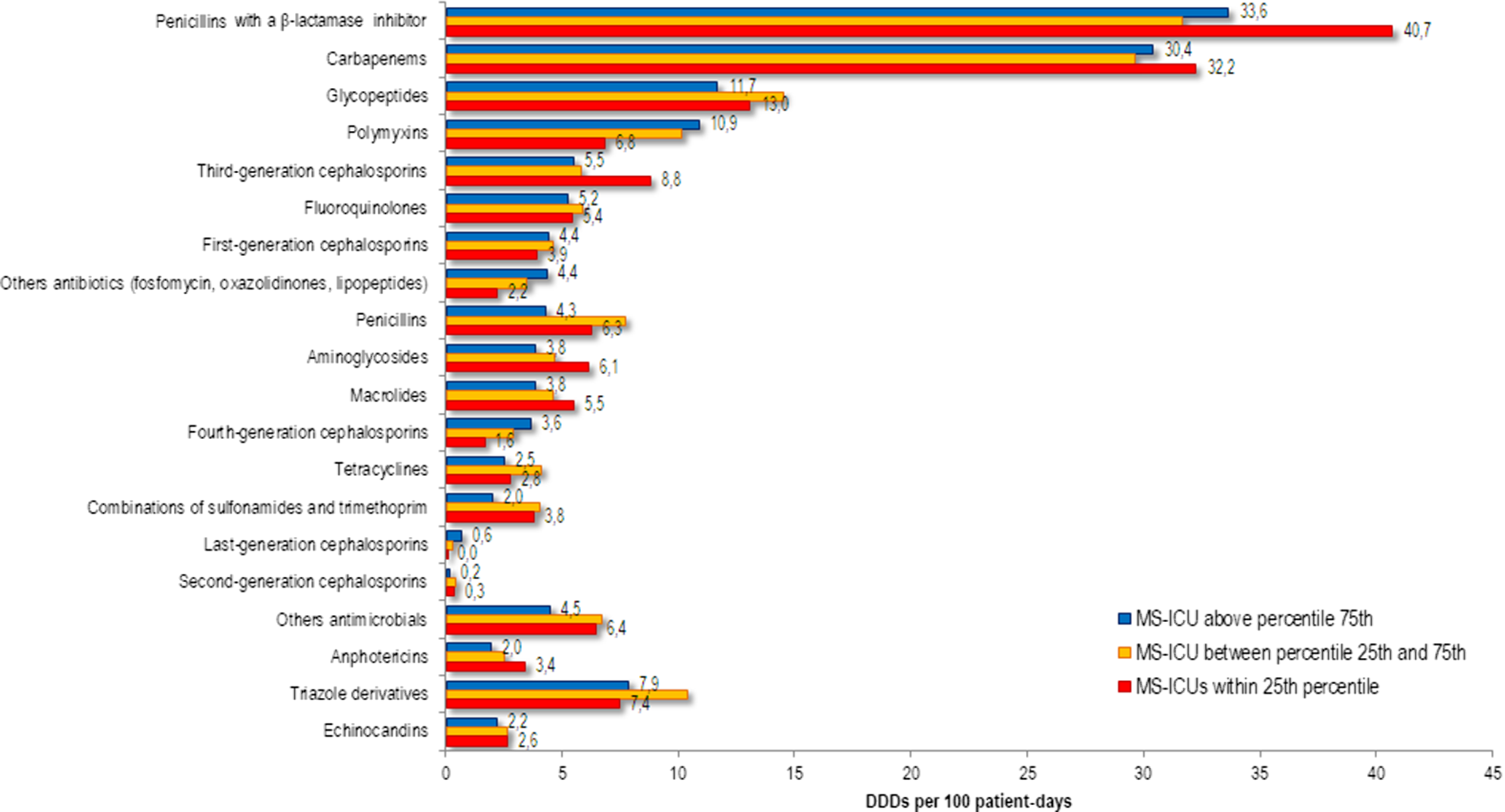
Fig. 2. Annual use of systemic antimicrobials in adult patients in medical-surgical ICU (MS-ICU) expressed as defined daily doses (DDDs) per 100 patient-days stratified by the global score of the final self-assessment.
The percentage of patients receiving at least 1 antimicrobial and the number of antimicrobials per patient on antimicrobial treatment were significantly lower in the MS-ICUs that reached the ≥75th percentile in comparison with those that remained within the 25th percentile at the final self-assessment (Table 3). The MS-ICUs that reached the ≥75th percentile had a significantly lower total antimicrobial consumption than those that remained in the 25th percentile (143.4 vs 159.4 DDD per 100 PD; P < .0001) (Table 3).
Table 3. Indicators Comparison Between MS-ICU Stratified by the Global Score of the Final Self-Assessment
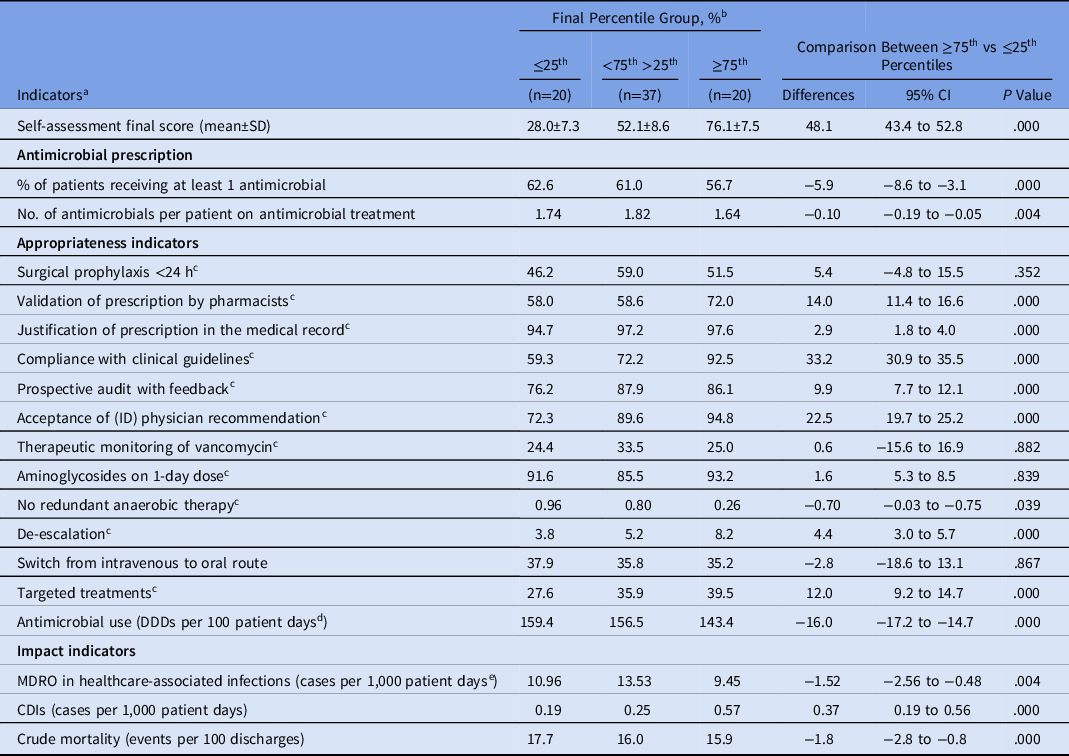
Note. MS-ICU, medical-surgical intensive care unit; CI, confidence interval; SD, standard deviation; ID, infectious diseases; DDD, defined daily dose; MDRO, multidrug-resistant organism; CDI, Clostridioides difficile infection.
a Cumulative indicators from July 2018 to June 2019.
b Percent unless otherwise indicated.
c Appropriateness rate: no. of prescriptions complying with the indicator × 100/total prescriptions of this category.
d Antimicrobial categories J01 and J02.
e MDROs: methicillin-resistant S. aureus; vancomycin-resistant Enterococcus; extended-spectrum β-lactamase Enterobacteriacea; carbapenem-resistant Enterobacteriacea; carbapenem-resistant P. aeruginosa and Acinetobacter spp.
The following appropriateness indicators performed better among MS-ICUs that reached the ≥75th percentile: validation of prescription by pharmacist, justification of prescription in the medical record, compliance with clinical guidelines, prospective audit with feedback, acceptance of ID physician recommendation, no redundant anaerobic therapy, de-escalation, and targeted treatments (Table 3). Surgical prophylaxis of <24 hours, therapeutic monitoring of vancomycin, aminoglycosides on 1-day dose, and switches from the intravenous to oral route did not show statistical difference.
Impact indicators
The cumulative impact indicators during the 12 months of the study showed that crude mortality and MDRO-HAIs were significantly lower in MS-ICUs in the ≥75th percentile than those in the 25th percentile. Only CDI was significantly higher in MS-ICUs in the ≥75th percentile (Table 3).
IPC strategies
MS-ICUs in the ≥75th percentile showed a significant higher frequency of IPC strategies implemented than those units within the 25th percentile (Table 4).
Table 4. Infection Prevention and Control Strategies Implemented at MS-ICUs Stratified by the Global Score of the Final Self-Assessment
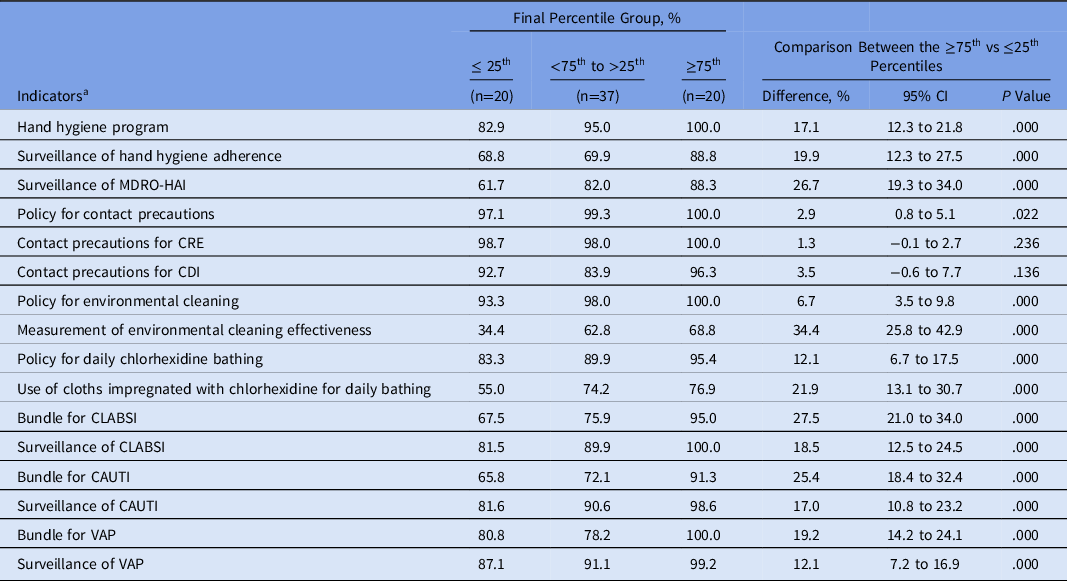
Note. MS-ICU, medical-surgical intensive care unit; CI, confidence interval; MDRO multidrug-resistant organisms: methicillin-resistant Staphylococcus aureus; vancomycin-resistant Enterococcus; extended-spectrum β-lactamase Enterobacteriacea; carbapenem-resistant Enterobacteriacea; carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter spp.; CDI, Clostridioides difficile infection. CLABSI, catheter-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia; HAI, healthcare-associated infection.
a Cumulative compliance from July 2018 to June 2019.
Discussion
This is the first multicenter study in Latin American MS-ICUs to evaluate antimicrobial prescription appropriateness, consumption and impact indicators in relation to the level of ASP development. Overall, 76.6% of the centers showed a significant improvement in their ASP scores. However, only 26.0% reached the 75th percentile in the final self-assessment, and 23.4% centers did not improve their global scores along the study.
The domain “leadership and coordination” scored highest at the final self-assessment, followed by “intervention strategies” and “monitoring.” “Education” was the domain with the lowest score. The latter represents an opportunity to develop and implement new strategies to improve education and training to prescribers and to patient and relatives. These findings are consistent with a previous study analyzing 4,184 US acute-care hospitals through the 2014 National Healthcare Safety Network Annual Hospital Survey, which reported that only 39.2% of institutions have an ASP meeting all 7 core elements. Reference Pollack, van Santen, Weiner, Dudeck, Edwards and Arinivasan30 In addition, written support or salary funding were significantly associated with having a comprehensive ASP. Reference Pollack, van Santen, Weiner, Dudeck, Edwards and Arinivasan30 In a cross-sectional study including 103 hospitals in Central and South America, the lack of hospital administration, lack of information technology support, and opposition from prescribers were stated as main barriers to the development of ASPs. Reference Howard, Pulcini and Levy Hara31 More recently, in a survey conducted in 27 Latin American hospitals, 40.7% of respondent hospitals did not have a written statement supporting an ASP, and 51.9% reported no financial support for ASP practices. In addition, only 26% of laboratories agreed to perform testing for MDROs, and only 40.7% of hospitals included education to prescribers on improving antibiotic use. Reference Muñoz, Motoa and Escandón-Vargas32
Our findings confirm that ASPs are often only partially implemented in Latin American hospitals. This issue represents a very important challenge because institutional support, interventions to optimizing antibiotic use, monitoring and reporting processes, as well as physician education, are necessary to implement an ASP effectively. Reference Dellit, Owens and McGowan15
The feasibility of doing a prevalence survey has been demonstrated in previous studies. Reference Quirós, Cabral and Bertuzzi25,Reference Versporten, Zarb and Caniaux27 This methodology has allowed hospitals in LMICs to assess antibiotic prescribing patterns and to collect information about antibiotic resistance for the first time. In that sense, measurement of appropriateness of antimicrobial prescription is essential for monitoring and reporting ASPs.
We observed lower antimicrobial resistance in the higher percentile group, and this finding could be related to lower consumption of antibiotics in the MS-ICUs that have a more comprehensive ASP. As in a previous study, we found that targeted treatments were more common for HAIs than CAIs. Reference Versporten, Zarb and Caniaux27 In addition, we observed a high rate of empiric use of carbapenems and vancomycin and a low rate of de-escalation. High rates of extended-spectrum β lactamase, methicillin-resistant Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae infections are leading to increased use of carbapenems, vancomycin, and polymyxins. Reference Versporten, Zarb and Caniaux27,Reference Guzman-Blanco, Labarca and Villegas33–Reference Escandón-Vargas, Reyesa, Gutiérrez and Villegasa35 These findings represent an opportunity to promote new and rapid diagnostic tests to improve empiric treatments. 36
In our study, MS-ICUs that reached the 75th percentile showed improvements in antimicrobial prescription appropriateness, antimicrobial consumption, and impact indicators compared to those that remained within the 25th percentile. Only CDI rates were significantly higher at MS-ICUs above the 75th percentile, likely related to better detection, as well as other risk factors such as consumption of proton pump inhibitors, which were not evaluated in this study. Reference Leonard, Marshall and Moayyedi37 In addition, higher compliance with IPC strategies was associated with ASPs that are more comprehensive.
This study has several limitations. Participation was voluntary, which may have biased participation to hospitals with an interest in antibiotic stewardship. The restriction of the study to MS-ICU may limit the generalizability of the results, and overrepresentation of Argentinean hospitals may limit more generalizable conclusions. The strengths of the project include the prospective study design, findings of improved antibiotic use, better outcomes, and description of a model that is practical for the LMIC setting.
Based on previous experience, we limited our project to adult inpatients admitted to MS-ICUs. We restricted the study to adult patients because of difficulties in performing data collection using days of therapy as the measure of antimicrobial consumption in many Latin American institutions. Although, DDD is a useful indicator in the adult population, it is not useful for pediatric patients. Reference Quirós, Cabral and Bertuzzi25,Reference Polk, Fox, Mahoney, Letcavage and MacDougall29 Another reason to constrain the study to MS-ICUs was to enhance feasibility and sustainability because a limited number of human resources are involved in development, implementation, and monitoring ASPs in many hospitals located in low- and middle- and lower-income countries. Reference Howard, Pulcini and Levy Hara31 In addition, AMR and the challenges of appropriateness of antimicrobial use are more frequent in ICUs than in general wards. Reference De Waele, Akova, Antonelli and Canton38,Reference Chiotos, Tamma and Gerber39
In summary, our results suggest that MS-ICUs with ASPs with higher global scores in the final-self assessment showed improved appropriateness and impact indicators and lower antimicrobial consumption than those with lower scores. MS-ICUs with more comprehensive ASPs showed significant improvement in antimicrobial utilization.
Acknowledgments
The coordinators of PROA-LATAM Project would like to thank all healthcare personal from MS-ICUs who were involved the study.
Financial support
This work was funded by Merck and Co. The study funder had no role in the study design or data collection, analysis, or interpretation.
Conflicts of interest
M.Z. has received grant support unrelated to this work from Pfizer, Moderna, Johnson and Johnson. and Merck and as a consultant for contrafact. No other authors have financial disclosures. All other authors report no conflicts of interest relevant to this article.
PROA-LATAM Project Group
Maria Paula Bernachea, Alejandra Arias (Clinica Conciencia, Neuquen, Argentina); Graciela Beatriz Sadino, Adriana Cuevas, Maria Luz Sosa (Clínica Privada Reina Fabiola, Cordoba, Argentina); Emilia Silvia Cohen, Laura Deaguilar, Agustina Franchi (Higa Eva Peron, San Martin, Argentina); Marisa Liliana Bernan, Maria Eugenia Russo, Josefina Saintout (Higa San Roque, La Plata, Argentina); Valeria Stradella, Lucy Anchiraico Galarza, Vanesa Kaneshiro (Hospital Aeronautico Cordoba, Cordoba, Argentina); Angelina Soledad Firpo, Julieta Milagros Buzzo (Hospital Alberto Caccavo, Coronel Suarez, Argentina); Cecilia Ezcurra, Sergio Ciotti, Liliana Fernandez Canigia (Hospital Alemán, CABA, Argentina); Gustavo Costilla Campero, Silvia Vera Amate Perez (Hospital Angel Padilla, Tucuman, Argentina); Viviana Novarese, Jimena Balladares, Analia Paula Boschi (Hospital Carlos Macia, Mar de Ajó, Argentina); Alejandra Viteri (Hospital Cesar Milstein, CABA, Argentina); Luz Maria Olivo, Sara Maria Amani, Juan Gonzalo Tomas (Hospital de Clinicas Presidente Nicolás Avellaneda, Tucuman, Argentina); Alejandra Cuello, Karina Cabral, Carina Vanessa Chirino (Hospital de Villa Mercedes, Villa Mercedes, Argentina); Claudio Amadio, Paola Avondet, Noelia Sofia Linero (Hospital Del Carmen, Godoy Cruz, Argentina); Silvina Villamandos, Mariana Paola Ballestero, Gabriel Isac Podkowa (Hospital Dr. Ramón Madariaga, Posadas, Argentina); Sandra Lambert, Marisabel Comas, Luciana Patricia Gonzalez (Hospital El Cruce, Florencio Varela, Argentina); Amelia Lucrecia Sosa (Hospital Escuela Gral San Martín, Corrientes, Argentina); Martin Hojman, Lucia Rusell, Marta Torres (Hospital General de Agudos “Bernardino Rivadavia,” CABA, Argentina); Sabrina Penco (Hospital Guillermo Rawson, Cordoba, Argentina); Laura Barcan, Corina Nemirovsky, María Inés Staneloni (Hospital Italiano de Buenos Aires, CABA, Argentina); Romina Bertuzzi, Cecilia Garelli, Maria Ines Jean Charles (Hospital Italiano de Cordoba, Cordoba, Argentina); Juan Ignacio Dapas, Cynthia Rivero, Andrea Vila (Hospital Italiano de Mendoza, Guaymallén, Argentina); Rosa Contreras, Laura Gil, Vanesa Yamila Sanchez (Hospital Marcial Quiroga, Capital, Argentina); Diego Marcelo Maurizi, Lucia Lamponi Tappata, Adolfo Quispe Laime (Hospital Municipal de Agudos Dr Leonidas Lucero, Bahia Blanca, Argentina); Lucia Esther Daciuk, Fernandez Adriana, Diego Alejandro Laplume (Hospital Nacional Prof. Alejandro Posadas, Ramos Mejia, Argentina); Mariana Rodriguez Raimondo (Hospital Néstor Kirchner, San Miguel de Tucuman, Argentina); Emilia Silvia Cohen (Hospital Privado Modelo, Vicente Lopez, Argentina); Maria Isabel Garzon (Hospital Privado Universitario de Cordoba, Cordoba, Argentina); Marcela Vera Blanch, Leandro Falleroni (Hospital Provincial de Rosario, Rosario, Argentina); Viviana Carballo (Hospital Raul Angel Ferreyra, Cordoba, Argentina); Adriana Manzur, Gabriela Rodriguez (Hospital Rawson, San Juan, Argentina); Leandro Gastón Ballatore, Natalia Mongelos, Lucia Villa (Hospital Regional de Ushuaia, Ushuaia, Argentina); Sandra Cappello, Delfina Platini (Hospital Samco Jaime Ferre, Rafaela, Argentina); Milton Decima (Hospital Señor Del Milagro, Salta, Argentina); Maria Laura Pereyra Acuña, Wanda Cornistein, Agustina Malvicini (Hospital Universitario Austral, Pilar, Argentina); Carolina Osuna (Hospital Zonal de Agudos “A. Eurnekian” Ezeiza, Ezeiza, Argentina); Lucrecia Soler Puy, Emilce Adriana Alarcón, María Cecilia Ramírez (Instituto de Cardiología de Corrientes Juana Francisca Cabral, Corrientes, Argentina); Yanina Nuccetelli (Instituto de Diagnostico, La Plata, Argentina); Maria Alejandra Urueña (Maternidad Nuestra Señora de Las Mercedes de Tucumán, San Miguel de Tucuman, Argentina); Gabriela Vidiella, Matias Lucero (Maternidad Suizo Argentina, CABA, Argentina); Luciana Gabriela Moya, Miriam Noemí Cheche, Alejandra Enciso (Policlinico Neuquen, Neuquen, Argentina); Maria Laura Seguro, Germán Arevalo, Ricardo Lamberghini (Sanatorio Aconcagua, Cordoba, Argentina); Federico Romero, Fernando Riera (Sanatorio Allende Nueva Cordoba, Cordoba, Argentina); Yanina Nuccetelli (Sanatorio Argentino, La Plata, Argentina); Adriana Manzur, Rosa Contreras, Sandra Roldan (Sanatorio Mayo, San Juan, Argentina); Maria Alejandra Urueña (Sanatorio Sarmiento, San Miguel de Tucuman, Argentina); Augusto Cordero Lobaton (Caja de Salud de La Banca Privada, La Paz, Bolivia); Maria Teresa Guillaux, Karla Cecilia Arteaga Coimbra, Tapia Torrez Juan Carlos (Clinica Foianini, Santa Cruz, Bolivia); Amanda Lima Dias Morais, Rafael Botelho Foernges (Hospital Ana Nery, Santa Cruz Do Sul, Brasil); Jane Margarete Costa (Hospital Astrogildo de Azevedo, Santa Maria, Brasil); Rochele Mosmann de Menezes, Marcelo Carneiro, Eliane Krumenauer (Hospital Santa Cruz, Santa Cruz Do Sul, Brasil); Cristine Pilati Pileggi Castro (Hospital Sao Vicente de Paulo, Passo Fundo, Brasil); Lessandra Michelin (Hospital Unimed Nordeste Rs, Caxias Do Sul, Brasil); Jose Manuel Munita, David Llanten, Maria de Los Angeles Spencer Sandino (Clínica Alemana de Santiago, Santiago, Chile); Pablo Tomás Valenzuela García, Carola Escobar Chinchilla, Sofia Palma Waldron (Hospital de La Dirección Previsional de Carabineros de Chile, Santiago, Chile); Jose Manuel Munita, Anne Sophie Peters, Sebastian Solar (Hospital Padre Hurtado, Santiago, Chile); Alexander Guerra Villafañe (Clinica Esensa, Cali, Colombia); Alexander Guerra Villafañe, Lady Viviana Acosta Castillo (Clínica La Estancia, Popayan, Colombia); Alexander Guerra Villafañe (Clínica Rey David, Cali, Colombia); Diana Catalina Zapata Cristancho, Aurora Ximena Castaneda Luquerna (Fundacion Cardioinfantil, Bogota, Colombia); Sandra Milena Gualtero Trujillo, Alejandro de La Hoz Gomez (Hospital Universtario San Ignacio, Bogota, Colombia); Washington Rene Aleman Espinoza (Hospital Alcivar, Guayaquil, Ecuador); Fausto Guerrero, Maria Jose Chicaiza (Hospital Carlos Andrade Marin, Quito, Ecuador); Ana Paulina Celi de La Torre, Marcela Bovera (Hospital de Los Valles, Quito, Ecuador); Cristina Moreno, Luis Felipe Melendez Carranza, Ana Gabriela Ortega Vallejo (Hospital Metropolitano, Quito, Ecuador); Juan Jose Romero, Yolanda Marina Espinosa Crespo, Sylvia Jeanneth Illicachi Analuisa (Hospital Vozandes, Quito, Ecuador); Carlos Garcia Cruz, Aquiles Eduardo Bowen Flores (Sociedad de Lucha Contra el Cancer), Guayaquil, Ecuador); Ana Belén Arauz (Clínica Hospital San Fernando, Ciudad de Panamá, Panamá); Ivan Toala, Livia Raquel Arosemena Peralta, Jose Anel Gonzalez (Complejo Hospitalario Dr. Arnulfo Arias, Ciudad de Panamá, Panamá); Ana Belén Arauz, Sánchez Alex, Joel Medina Jil (Hospital Santo Tomás, Ciudad de Panamá, Panamá); Jorge Alave, Jussara Huamani, Daniel Saito Roncal (Clinica Good Hope, Breña, Perú); Eddie Alessandro Angles Yanqui, Jorge Dante Florez Arce, Rosa Liduvina Teran Robles (Hospital Nacional Arzobispo Loayza, Lima, Perú); Luis Cuellar, Alexis Holguin Ruiz, Juan Velarde Marca (Instituto Nacional de Enfermedades Neoplasicas, Lima, Perú); Karina Tenaglia, Grisel Rodriguez, Daniela Tomera (CAMS IAMPP, Mercedes, Uruguay); Andrea Iturralde, Adriana Dorrego, Lilián Morales (COMEF IAMPP, Florida, Uruguay); German Echenique, Alicia Ottonelli, Patricia Zitto (COMEPA IAMPP, Paysandu, Uruguay); Isabel Bartaburu, María Alejandra Calvo Rothfuss, Jhony Gustavo Perin Tamagno (Sanatorio Panamericano, Salto, Uruguay); Isabel Bartaburu, Miriam Raquel Diaz Ana María Félix Sosa (Sanatorio Uruguay, Salto, Uruguay).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.80