1. Introduction
Thirty years ago, in this journal, W. R. Engels (Reference Engels1979) introduced the term ‘cytotype’ to describe the combination of chromosomal and cytoplasmic factors that determine whether or not the offspring of crosses between different strains of Drosophila will exhibit a syndrome of abnormalities called hybrid dysgenesis. These abnormalities are restricted to the germ line and include temperature-sensitive sterility, high frequencies of mutation and chromosome breakage, chromosome non-disjunction and transmission ratio distortion. The syndrome of hybrid dysgenesis had been described two years earlier in a landmark paper (Kidwell et al., Reference Kidwell, Kidwell and Sved1977) that broadly classified Drosophila strains into two main categories: P strains, which contribute paternally to the induction of dysgenesis, and M strains, which contribute maternally to it. Thus, when P males are crossed to M females, the offspring are dysgenic, but when the reciprocal cross is performed, the offspring are normal. Engels' genetic analysis of this phenomenon led him to conclude that P strains harbour chromosomal factors – ‘P factors’ – that induce dysgenesis, and that these same factors also have the ability to repress dysgenesis. Somehow, the P factors are autoregulatory.
The phenomenon of P-M hybrid dysgenesis was elucidated when Engels' P factors were found to be transposons, now called P elements (Bingham et al., Reference Bingham, Kidwell and Rubin1981). These elements transpose through the action of a transposase encoded by the structurally complete members of the P-element family (Engels, Reference Engels1984; Karess & Rubin, Reference Karess and Rubin1984). Other, structurally incomplete P elements cannot produce this enzyme, although they can be transposed as long as a transposase-producing complete P element is present somewhere in the genome. P transposition is restricted to the germ line because the intron between exons 2 and 3 in the complete P element (the ‘2–3 intron’) is removed from the pre-mRNA only in that tissue (Laski et al., Reference Laski, Rio and Rubin1986).
P transposition is largely repressed in P strains and in the offspring of crosses between P females and M males; however, in the offspring of crosses between P males and M females, it occurs frequently. Engels (Reference Engels1979) called the cellular state that permits transposition the M cytotype, and he called the state that represses it the P cytotype. Although the cytotype of a fly ultimately depends on the presence or absence of P elements on its chromosomes, in the short term it depends on the cytotype of its mother. This short-term maternal effect explains why dysgenesis occurs only in the offspring of one of the two reciprocal crosses between P and M strains. The offspring of M females inherit the M cytotype, which permits the P elements they inherit from their fathers to be activated, whereas the offspring of P females inherit the P cytotype, which represses the P elements they inherit (Engels, Reference Engels, Berg and Howe1989).
It now appears that the cytotype-mediated repression of hybrid dysgenesis involves small RNAs that interfere with the production of the P transposase (Brennecke et al., Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). These RNAs are known to be generated from P elements that have fortuitously inserted in the telomere-associated sequences (TAS) near the left end of the X chromosome. An important series of studies by Stéphane Ronsseray, Dominique Anxolabéhère and their colleagues had previously established that these types of P insertions repress hybrid dysgenesis (Ronsseray et al., Reference Ronsseray, Lehmann and Anxolabéhère1991, Reference Ronsseray, Lemaitre and Coen1993, Reference Ronsseray, Lehmann, Nouaud and Anxolabéhère1996, Reference Ronsseray, Marin, Lehmann and Anxolabéhère1998; Marin et al., Reference Marin, Lehmann, Nouaud, Izaabel, Anxolabéhère and Ronsseray2000; Josse et al., Reference Josse, Teysset, Toideschini, Sidor, Anxolabéhère and Ronsseray2007). Other studies have shown that small regulatory RNAs form complexes with the Piwi class of proteins, which are important components of an RNA interference (RNAi) mechanism (Brennecke et al., Reference Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam and Hannon2007). These kinds of RNAs are therefore called Piwi-interacting RNAs or simply piRNAs.
A majority of the piRNAs produced from P insertions in the TAS of XL are antisense to P mRNAs. Sense piRNAs are also produced from these P insertions, as well as from other, non-telomeric P elements scattered around the genome (Brennecke et al., Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). The genesis of these complementary classes of piRNAs is not fully understood. However, it is thought that the two classes mutually reinforce each other's production through a cyclical process called the ‘ping-pong’ mechanism (Brennecke et al., Reference Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam and Hannon2007, Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). Briefly, antisense transcripts derived from the telomeric P elements generate antisense piRNAs, which then target for destruction sense P mRNAs derived from the telomeric or other P elements. Fragments of these degraded mRNAs become sense piRNAs, which in turn target newly generated antisense P transcripts to produce more antisense piRNAs. As this cyclical process continues, sense and antisense piRNAs accumulate in the germ line and P mRNAs, including those that encode the transposase, are destroyed. Without mRNA to make the transposase, the members of the P element family remain quiescent and hybrid dysgenesis is prevented. The ping-pong mechanism therefore implies that telomeric and non-telomeric P elements – or more precisely, the RNAs they produce – interact synergistically to repress dysgenesis.
This paper investigates the genetic and molecular underpinnings of the ping-pong mechanism. The analysis builds on previous observations that the telomeric P elements known as TP5 and TP6 interact with dispersed, non-telomeric P elements to bring about strong repression of hybrid dysgenesis (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a). TP5 is a 1·8 kilobase (kb)-long P element inserted in one of the repeat sequences within the TAS of XL and TP6 is a 1·9 kb-long P element inserted at the same site (Stuart et al., Reference Stuart, Haley, Swedzinski, Lockner, Kocian and Simmons2002). Both of these elements were isolated from flies derived from natural populations in the Midwestern United States – TP5 from a population in Madison, Wisconsin, and TP6 from a population in Mt. Carmel, Illinois. By themselves, these elements repress hybrid dysgenesis to some degree, but in combination with dispersed, non-telomeric P elements – for example, the P elements from the strain known as Muller-5 Birmingham, their repression power is significantly strengthened. This strengthening is not due to a simple additive effect of the Birmingham P elements, but rather, to a genuine synergism between the telomeric and non-telomeric P elements (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a).
In this paper, we address several questions about this synergism. First, is the maternal effect associated with the telomeric P elements sufficient to establish a synergistic interaction with the non-telomeric P elements? Second, can synergistic repression of hybrid dysgenesis persist after the telomeric P elements are removed from the genotype? Third, what effect does synergism have on P-element mRNA? Fourth, do mutations in genes relevant to cytotype prevent the establishment of synergism, and fifth, do these mutations disrupt synergism that has already been established?
2. Materials and methods
(i). Drosophila stocks and husbandry
Information about the Drosophila stocks can be found in Lindsley & Zimm (Reference Lindsley and Zimm1992), the Flybase website, and references cited in the text. The isolation and initial analysis of the telomeric P elements TP5 and TP6 is described in Stuart et al. (Reference Stuart, Haley, Swedzinski, Lockner, Kocian and Simmons2002) and maps of these elements are presented in Jensen et al. (Reference Jensen, Stuart, Goodpaster, Goodman and Simmons2008). All stocks carrying these elements are marked with the white eye (w) mutation, which is tightly linked to the XL telomere. Independently maintained derivatives of the original TP5 and TP6 stocks were used in the experiments reported here; they are denoted TP5-1, TP5-2, TP6-1 and TP6-2.
Stocks with TP5 or TP6 and autosomal P elements from the Muller-5 Birmingham strain were developed from the TP5-1 and TP6-1 strains (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a). Even though Muller-5 Birmingham contains numerous P elements, it has the M cytotype; it is a so-called M′ strain (Bingham et al., Reference Bingham, Kidwell and Rubin1981). Throughout this paper, the autosomal Birmingham P elements are denoted by the abbreviation Birm. None of these elements is telomeric or encodes the P transposase.
Stocks carrying a telomeric P element and an autosomal mutation relevant to P-regulation were constructed in a two-step process. First, the Gla mutation and the CyO balancer chromosome were introduced into the TP5-2 and TP6-2 stocks through a series of backcrosses to create TP (either TP5 or TP6); CyO/Gla stocks. Then females from these stocks were crossed to CyO/mutation males to produce TP; CyO/mutation males, which were then backcrossed to TP; CyO/Gla females. The TP/TP; CyO/mutation daughters and TP; CyO/mutation sons of this last cross were then intercrossed to establish the TP; CyO/mutation stocks. The mutations incorporated into these stocks were in the genes aubergine (aub), piwi, or Suppressor of variegation 205 [Su(var)205]. Because these mutations are either lethal or sterile in homozygous condition, they had to be maintained with the CyO balancer chromosome. This balancer also carries a P transgene, P(SB)7, which is not relevant to the experiments reported here.
After the TP; CyO/mutation stocks had been synthesized, a set of follow-up control stocks were created from them by removing the mutations. The procedure was to cross TP; CyO/mutation females with males hemizygous for the FM6 balancer X chromosome. The TP/FM6; CyO/+ daughters and TP; CyO/+ sons of these crosses were then mated to produce TP/TP; CyO/+ females and TP; CyO/+ males, which were intercrossed to establish mutation-free control stocks that were subsequently maintained by selecting flies that carried the CyO chromosome in each generation. The TP; CyO/mutation stocks and the mutation-free stocks derived from them were maintained for more than one year before being used in any experiments.
Experimental cultures were reared in shell vials on a standard cornmeal–molasses–yeast medium at 25°C, unless otherwise stated. Stock cultures were maintained in shell vials or in half-pint milk bottles at 18–21°C.
(ii). Assay for gonadal dysgenesis (GD)
When mobilized, P elements can induce a form of sterility known as gonadal dysgenesis (GD). This condition results from extensive destruction of the germ-line tissues – so extensive that gametes are not produced. To screen for GD, we squashed samples of females between two glass slides and looked for eggs. A solution of green food colouring provided a background against which the eggs could be scored. Females without any eggs were scored as dysgenic.
The flies to be scored were produced by crossing females of a test genotype to males from the Harwich P strain, which is marked with w (Kidwell et al., Reference Kidwell, Kidwell and Sved1977). The test females were initially mass mated at 21°C. After three days, they were placed in separate culture vials, which were incubated at 29°C, a temperature that increases the frequency of GD. On day 11, all the offspring were transferred to a holding vial, where they were allowed to mature for two days. Then, as many as 20 female offspring of each segregating genotype were scored for the presence or absence of eggs using the squashing technique.
(iii). Statistical analyses
The frequency of GD was calculated independently for each replicate culture in a group, and the unweighted average frequency of GD among the replicates was used to characterize that group. The standard error (SE) for the group was computed empirically from the variance among replicates. Statistical differences between groups were assessed by performing t or z tests.
(iv). RNA isolation and reverse transcription (RT)-PCR
RNA was isolated from groups of 20 virgin females using TRIZOL (Invitrogen) according to the supplier's instructions. The RNA was reverse transcribed into cDNA using the ThermoScript reverse transcriptase (Invitrogen) and an oligo-dT primer, and the resulting cDNA was amplified by the PCR using appropriate oligonucleotide primers and temperature profiles. The detailed methods for RT-PCR and the primer sequences are given in Jensen et al. (Reference Jensen, Stuart, Goodpaster, Goodman and Simmons2008). PCR products were analysed in 1% agarose gels by electrophoresis at 70 volts.
3. Results
(i). Establishing synergism between telomeric and non-telomeric P elements
The establishment of synergistic interactions between the telomeric P elements TP5 or TP6 and non-telomeric P elements from the Birmingham strain was investigated in a multi-generation experiment in which each of the telomeric P elements was represented by two independently maintained stocks; another stock that did not carry any P elements was included as a control.
The first stage of the experiment assessed the ability of TP5 and TP6 to repress GD in the absence of any interacting, non-telomeric P elements. Homozygous TP5 or TP6 females were crossed to wild-type males from an M strain to produce F1TP/+ females, which were then crossed to males from the Harwich w P strain to induce GD. The two types of daughters of this last cross – those with and those without the telomeric P element – were scored separately for dysgenesis. The results, summarized on the left side of Table 1, show that dysgenesis was repressed – although to different degrees – in flies from three of the stocks: TP5-2, TP6-1 and TP6-2. Other analyses have suggested that variation in the strength of repression might be due to differences in the structure of the XL telomere (Thorp et al., Reference Thorp, Chapman and Simmons2009). The results of this stage of the experiment also indicate that repression of dysgenesis was equivalently strong in the two types of daughters that were scored. Repression in the daughters that did not inherit a telomeric P element clearly shows that P-element regulation involves a maternal effect.
Table 1. Synergistic repression of GD by telomeric and Birm P elements

a F1TP/+ females were produced by crossing wild-type Samarkand males, which lack P elements, to females homozygous for the telomeric P element TP5 or TP6 and the tightly linked eye colour marker w. The two classes of F2 females were produced by crossing F1TP/+ females to w; Birm males. These two classes were distinguished by following the segregation of the eye colour marker; the F2 females with white eyes [class (1)] carried the TP element and those with red eyes [class (2)] did not. Control flies were produced by using females that did not carry a telomeric P element in the initial cross. Tests for repression of GD were carried out by crossing samples of F1 and F2 females to Harwich w males, as described in the ‘Materials and methods’ section. For the F1 females, daughters with and without the telomeric P element were scored separately.
b All the stocks were marked with w. The TP6-1 stock also had the X-linked markers m and f.
c Unweighted mean percentage of GD±SE.
The next stage of the experiment assessed the ability of the telomeric P elements, or their maternal effects, to repress GD through interactions with non-telomeric P elements from the Birmingham strain. A sample of F1TP/+ females from the initial crosses were mated to males from a strain with the Birmingham autosomes to produce two classes of F2 females: (1) TP/+; Birm/+ and (2) +/+; Birm/+. These females were then crossed to Harwich w males to induce GD. In the class (1) F2 females, there is a possibility for the telomeric P element to interact with the Birm P elements, whereas in the class (2) F2 females, no such interactions can occur; however, in this class of females the Birm P elements might interact with a maternal effect of the telomeric P element.
The data from this stage of the experiment are summarized on the right side of Table 1. With all four TP stocks, the daughters of the class (1) F2 females repressed GD, and the level of repression was greater than that seen in the previous generation. The difference between the two generations indicates that repression by the telomeric P elements is strengthened by the non-telomeric Birm P elements. Furthermore, because the Birm P elements have no regulatory power by themselves (see the results with the control strain w), this enhanced repression is evidence for synergism between the telomeric P elements and the Birm P elements. By contrast, the daughters of the class (2) F2 females did not repress dysgenesis. The absence of repression in these flies indicates that even though telomeric P elements act through a maternal effect, this effect does not interact with the Birm P elements to regulate the P element family.
(ii). Persistence of synergistic regulation
We performed another multi-generation experiment to determine if synergistic regulation could persist after the telomeric P elements were removed from long-established TP; Birm stocks that had previously been shown to repress GD almost completely (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a). The experiment began by crossing females from TP5; Birm and TP6; Birm stocks to males from a +; Birm stock to produce F1TP/+; Birm females, which had one dose of the telomeric P element and two doses of the Birm P elements. One sample of these females was crossed to Harwich w males to induce GD and a second sample was crossed to males from a w; Birm stock to produce two classes of F2 females: (1) TP/w; Birm (with white eyes) and (2) +/w; Birm (with red eyes). Both classes of females had two doses of the Birm P elements but only the class (1) females carried a telomeric P element. Samples of each of these classes of F2 females were crossed to Harwich w males to induce GD. A sample of the class (2) F2 females was also crossed to +; Birm males to produce F3 females that, like their mothers, carried two doses of the Birm P elements but no telomeric P element. These females were then crossed to Harwich w males to induce GD. This overall scheme was also applied to a w; Birm stock, which served as a control. The data from all three stages of the experiment are summarized in Table 2.
Table 2. Repression of GD in the descendants of TP; Birm stocks
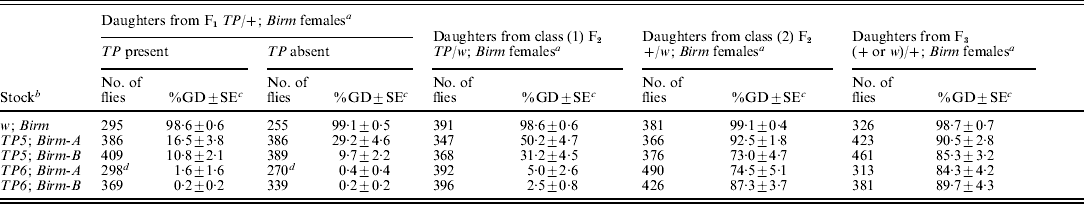
a The F1TP/+ females were produced by crossing TP; Birm females to +; Birm males. The two classes of F2 females were produced by crossing F1TP/+ females to w; Birm males. These two classes were distinguished by following the segregation of the eye colour marker w, which is tightly linked to the TP in each of the stocks; the F2 females with white eyes [class (1)] carried the TP and those with red eyes [class (2)] did not. The F3 females were produced by crossing F2 +/w; Birm females to +; Birm males. Control flies were produced by using females that did not carry a telomeric P element in the initial cross. Tests for repression of GD were carried out by crossing samples of F1, F2 and F3 females to Harwich w males, as described in the ‘Materials and methods’ section. For the F1 females, daughters with and without the telomeric P element were scored separately.
b All stocks were marked with w. The TP6 stocks also had the X-linked markers m and f.
c Unweighted mean percentage of GD±SE.
d Data collected from a total of 32 vials; all other data came from a total of 25 vials.
In the first stage of the experiment, dysgenesis was repressed strongly in the flies derived from the TP; Birm stocks – less than 2% GD among the daughters of the F1TP6/+; Birm females and between 10 and 29% GD among the daughters of the F1TP5/+; Birm females. By contrast, the flies derived from the w; Birm control strain did not repress GD at all. These results indicate that by themselves, the Birm P elements have no repression ability; however, in combination with either of the telomeric P elements, they bring about strong repression. These results also indicate that repression involved a maternal effect. The flies that did not inherit a telomeric P element repressed GD about as well as those that did.
The next stage of the experiment had two components. First, we scored for repression of GD in the daughters of the class (1) F2TP/w; Birm females; however, owing to the presence of the w marker in both of the X chromosomes, we could not distinguish between the daughters that inherited the telomeric P element and those that did not. For the daughters of the class (1) F2TP6/+; Birm females, repression was strong – only slightly less than that seen in the previous generation. For the daughters of the class (1) F2TP5/+; Birm females, it was less than that seen previously (50% GD vs. 16–29%, and 31% GD vs. 10%). Because the F1 and F2 females used in these tests were genotypically equivalent – both carried a single dose of the TP and two doses of the Birm P elements, this difference suggests that repression in the daughters of the F1 females was boosted by a grand-maternal effect of the TP and Birm P elements in the females of the initial cross.
The second component of this stage of the experiment involved the class (2) F2 +/w; Birm females, which did not carry a telomeric P element. These females provided an opportunity to see if the ability to repress dysgenesis could persist after the telomeric P element was completely removed from the genotype. The incidence of dysgenesis ranged from 73 to 92% in the daughters of these females, but in the corresponding controls, it was 99%. Thus, a weak but significant ability to repress dysgenesis persisted after the telomeric P element had been removed from the genotype. This observation confirms a previous report of repression persisting in flies derived from TP; Birm stocks (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a).
In the last stage of the experiment, we measured repression ability in the daughters of F3 females that had lost the telomeric P element two generations earlier. The incidence of dysgenesis in these flies was high (84–90%), but it was significantly less than that in the control (99%). Thus, some ability to repress dysgenesis persisted one more generation after the telomeric P element had been removed from the genotype.
(iii). Effect of synergistic regulation on germ-line P RNA
To investigate synergistic regulation at the molecular level, we used RT-PCR to assess the abundance of a specific P-element mRNA in the germ lines of females that carried TP6 and a single dose of the Birm P elements. The specific mRNA that we monitored was produced by H(hsp/TP5)D, an autosomal insertion of a hobo transgene that contains the TP5 element fused to a heat-shock-inducible promoter (Jensen et al., Reference Jensen, Stuart, Goodpaster, Goodman and Simmons2008). However, because the TP5 element also has its own promoter, no heat shock was needed (or used) to generate RNA from this element. This transgene was chosen because none of the Birm P elements is a TP5 element. Thus, in females that carry both the transgene and the Birm P elements, the H(hsp/TP5)D transgene is the unique source of TP5 RNA. The females from which the RNA was extracted were produced by crossing w; H(hsp/TP5)D males to TP6; Birm females from two independently maintained TP6; Birm stocks; we obtained two RNA samples from the TP6/+; H(hsp/TP5)D/Birm daughters of each cross. RNA was also extracted from females obtained from control crosses between w; H(hsp/TP5)D males and females from three different stocks: (1) w (no TP6 and no Birm P elements, two independent RNA samples), (2) w; Birm (no TP6, two independent RNA samples) and (3) TP6 (no Birm P elements, four independent RNA samples). In parallel with these crosses, we also carried out genetic tests for repression of GD by crossing females from all five stocks to Harwich w males.
The results of the genetic tests for repression of dysgenesis are summarized in Table 3. Both of the TP6; Birm strains were powerful repressors. The TP6 strain – the same one used to create the TP6; Birm strains – was also a strong repressor, but not as strong as either of the TP6; Birm strains. The w and w; Birm strains did not repress dysgenesis at all. For comparison, we also tested TP5 and TP5; Birm strains. The latter was a powerful repressor, whereas the former was less so.
Table 3. Repression of GD by TP and TP; Birm stocks

a All TP6 stocks were marked with w, m and f.
b Unweighted mean percentage of GD±SE.
The results of the RT-PCR analysis are given in Fig. 1. Panel (A) shows the RT-PCR product obtained in amplifications with primers specific for mRNA from the aub gene, which is known to be expressed in the female germ line. Because there are no obvious differences in the amount of this product, the samples appear to have had roughly equal amounts of input mRNA. Panel (B) shows the RT-PCR product obtained in amplifications with primers specific for TP5 mRNA synthesized in the germ line. The specificity of this amplification arises from the fact that one primer spanned the deletion breakpoint in the TP5 element and the other spanned the 2–3 intron, which is removed from P-element RNA only in the germ line. In this RT-PCR, the samples from the w; H(hsp/TP5)D/+ and w; H(hsp/TP5)D/Birm flies clearly generated the expected product. By contrast, the samples from the TP6/w; H(hsp/TP5)D/+ and TP6/w; H(hsp/TP5)D/Birm flies either did not yield this product, or yielded lesser amounts. Note that the samples from these latter two genotypes were analysed side-by-side to permit pair-wise comparisons. In three of the four pairs, there was less of the TP5 RT-PCR product in the TP6/w; H(hsp/TP5)D/Birm sample.

Fig. 1. RT-PCR analysis of mRNAs from females heterozygous for the telomeric P element TP6 (denoted ‘T’), the non-telomeric Birm P elements (denoted ‘B’) and the H(hsp/TP5)D transgene (present in all samples). Control females (denoted ‘C’) did not carry either TP6 or the Birm P elements. Each RNA sample was obtained independently. PCR primers and reaction conditions are detailed in Jensen et al. (Reference Jensen, Stuart, Goodpaster, Goodman and Simmons2008). A plus indicates where an aliquot from a sample has been reversed transcribed, and a minus indicates where it has not. (A) Amplification over 25 cycles using primers Aub-d and Aub-u to detect an 848-bp product from aubergine mRNA. (B) Amplification over 30 cycles using primers TP5-d and PΔ2/3-u to detect a 471-bp product from TP5 mRNA transcribed in the germ line from the H(hsp/TP5)D transgene.
The combined genetic and molecular analysis suggests that the amount of germ-line TP5 mRNA is inversely related to the repression ability of the parent stock. It was most abundant in the females derived from the w and w; Birm stocks, which had no repression ability, and least abundant in the females derived from the TP6; Birm stocks, which had very strong repression ability. This inverse relationship suggests that repression of P activity involves either targeted destruction of P-element mRNA or blockage of P mRNA synthesis, and that when this repression is strong, as in genotypes that contain both telomeric and Birm P elements, P-element mRNA is largely eliminated from the germ line.
(iv). Effect of mutations on the establishment of synergistic regulation
We tested mutations in three genes – aub, piwi and Su(var)205 – for an impact on the synergistic regulation of P elements. The first part of this analysis determined if any of these mutations, when maternally transmitted, could prevent the establishment of synergism between a telomeric P element and the Birm P elements. Females from stocks that were homozygous for either TP5 or TP6 and heterozygous for the mutation under test were crossed to +; Birm males to produce F1TP/+; Birm/mutation females, which were then crossed to Harwich w males to induce GD in their daughters. The results are summarized in Table 4.
Table 4. Effects of mutations on the establishment of synergism between TP and Birm P elements

a The F1 females were produced by crossing TP w; CyO/mutation females with +; Birm males. These F1 females were then crossed to Harwich w males to induce GD in their daughters.
b Unweighted mean percentage of GD±SE.
The control flies, which carried the Gla mutation, strongly repressed dysgenesis, regardless of whether or not their daughters inherited the telomeric P element. This strong repression was clearly due to synergism between the telomeric and Birm P elements because other tests (not shown) demonstrated that either of these components by themselves did not repress (>96% GD). Four mutations – aub ΔP−3a, aubQC42, piwi1 and Su(var)2054 – appeared to block the establishment of synergism between the telomeric and Birm P elements. The Su(var)2054 mutation had the most telling effect – completely blocking TP5 synergism and almost completely blocking TP6 synergism. The two aub mutations had less telling effects and they appeared to block TP5 and TP6 synergism differently; aub ΔP−3a was a moderate blocker of TP5 synergism and a strong blocker of TP6 synergism, whereas aubQC42 was a strong blocker of TP5 synergism and a weak blocker of TP6 synergism. The results with the two piwi mutations showed that only piwi1 blocked synergistic repression of GD, and it did so only with the TP5 element.
To follow up these findings, we carried out the same type of experiment with TP; CyO/+ stocks from which the aub, piwi and Su(var)205 mutations had been removed many generations earlier. The purpose was to see if the various mutational blocks to synergism could be lifted, and the results, summarized in Table 5, show that, with one exception, they were. The TP5 and TP6 stocks that had lost the aubQC42, piw1, and Su(var)2054 mutations recovered their ability for synergistic repression of GD completely. The TP6 stock that had lost the aub ΔP−3a mutation also substantially recovered its synergistic repression ability. Only the TP5 stock that had lost the aub ΔP−3a mutation failed to recover the capacity for synergism. These results suggest that the impairments to synergistic repression documented in Table 4 were genuinely due to the mutations in the stocks and not to some other factor such as a change in the structure of the XL telomere. However, such factors might explain why the TP5 stock that had lost the aub ΔP−3a mutation did not recover the capacity for synergism; they might also explain the reduced capacity for synergism in the TP5 stock that had lost the piwi2 mutation (29–38% GD compared with 15–19% GD in the TP5 stock that carried the piwi2 mutation).
Table 5. Synergistic repression by TP stocks from which aub, piwi or Su(var)205 mutations had been removed

a The F1 females were produced by crossing TP w; CyO/(mutation) females with +; Birm males. These F1 females were then crossed to Harwich w males to induce GD in their daughters.
b Unweighted mean percentage of GD±SE.
(v). Effect of mutations on established synergistic regulation
Another part of this analysis involved ascertaining if any of the aub, piwi or Su(var)205 mutations could, when paternally transmitted, disrupt synergism that had already been established between a telomeric P element and the Birm P elements. The experiment to address this issue utilized long-standing TP; Birm stocks that had strong abilities to repress GD. Females from these stocks were crossed to males that were heterozygous for the mutations under test. The F1TP/+; Birm/mutation females were then crossed to Harwich w males to induce GD in their F2 daughters. This scheme was also applied to a control w; Birm stock that lacked a telomeric P element. As expected, nearly all the daughters from the crosses with this last stock were dysgenic (data not shown). The results from the crosses with the TP; Birm stocks are summarized in Table 6.
Table 6. Disruption of synergism between TP and Birm P elements by mutations

a The F1TP/+; Birm/mut females were produced by crossing TP5; Birm-B or TP6; Birm-A females with +; (CyO or Cy Roi)/mutation males. The control F1 females (no mutation) were produced by crossing these TP; Birm females with wild-type males from the Samarkand M strain. All the F1 females were crossed to Harwich w males to induce GD in their daughters.
b Unweighted mean percentage of GD±SE.
For the most part, the frequency of dysgenesis in the F2 females derived from the TP5; Birm and TP6; Birm stocks was low, even when these females did not inherit a telomeric P element. There were, however, two exceptions to this general pattern. The Su(var)2054 mutation disrupted repression by the TP5-Birm and TP6-Birm combinations, although not as dramatically as it prevented the establishment of repression by these combinations (Table 4), and the piwi1 mutation disrupted repression by the TP5-Birm combination. Note, however, that neither of the aub mutations had an effect on repression by either of the TP-Birm combinations. Thus, these mutations do not disrupt TP-Birm P synergism that has already been established even though they do significantly impair its establishment in the first place (see Table 4), presumably through a maternal effect (Simmons et al., Reference Simmons, Ryzek, Lamour, Goodman, Kummer and Merriman2007b). Such an effect would not be exerted on the F1 females that were tested to obtain the data in Table 6 because these females had inherited their aub mutations paternally.
4. Discussion
The ping-pong mechanism has been proposed as a way in which Drosophila mount a strong defence against potentially destructive transposable elements (Brennecke et al., Reference Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam and Hannon2007, Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). This mechanism is thought to be initiated by piRNAs that are antisense to transposon mRNAs. Interactions between these piRNAs and mRNAs produce a population of sense piRNAs, which then interact with transposon antisense RNAs to produce more antisense piRNAs. As this process cycles back and forth, sense and antisense piRNA populations increase and transposon mRNAs are largely destroyed. Transposon movement is thereby repressed.
Antisense piRNAs are generated by special loci – for example, the TAS repeats in the telomere of XL. P elements inserted at this site produce antisense piRNAs with P sequences, and these piRNAs are transmitted through the egg (Brennecke et al., Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). In the offspring, maternally transmitted antisense piRNAs may provide the basis of a defence against paternally inherited P elements, and thus may account for the strictly maternal repressing effect that is associated with telomeric P elements. However, experiments in which dysgenesis was induced by a single paternally inherited P element indicate that by itself the strictly maternal effect of a telomeric P element is insufficient to repress dysgenesis strongly (Thorp et al., Reference Thorp, Chapman and Simmons2009). It therefore seems likely that strong repression requires ping-pong cycling between maternally inherited antisense piRNAs and RNAs produced by the plethora of P elements transmitted by the father in a typical dysgenic cross.
This cycling very likely occurs in the daughters of females that carry a telomeric P element such as TP5 or TP6 and males from a P strain such as Harwich, even if the daughters do not inherit the telomeric P element. Evidently, at least one of the Harwich P elements provides the antisense P transcripts needed to sustain the ping-pong mechanism – a reasonable assumption given that Harwich has the P cytotype. The ping-pong mechanism would also be expected to occur in flies that carry maternally inherited telomeric P elements and paternally inherited Birm P elements. The telomeric P elements would be sources for antisense piRNAs and the Birm P elements would be sources for sense piRNAs. Females with both TP and Birm P elements would presumably be able to pass on both types of piRNAs to their offspring, and this maternal endowment would be expected to provide the basis for a strong defence against paternally transmitted P elements from a strain such as Harwich. The strength of this defence would in part arise from the ability of these piRNAs to jumpstart the ping-pong mechanism in the offspring when the Harwich P elements are expressed. Amplification of P-specific piRNAs during ping-pong cycling in TP; Birm females therefore plausibly explains the strong repression of dysgenesis in their daughters.
Although telomeric and Birm P elements interact synergistically to repress dysgenesis, the maternal effect associated with the telomeric P elements is not by itself sufficient to establish synergism with the Birm P elements. Evidently, a telomeric P element is needed to sustain the ping-pong amplification of piRNAs when the Birm P elements are introduced into the genotype through a cross between TP females and Birm males. However, once established, the ability to repress dysgenesis persists for at least one, and possibly two, generations after the telomeric P elements are removed from the genotype. This persistence suggests that some of the Birm P elements may be inserted in minor loci capable of generating antisense piRNAs, and that these loci are activated by piRNAs from the telomeric locus. Indeed, telomeric P elements that have been silenced by passing through males can be reactivated in females, and this reactivation is facilitated by the maternal and zygotic effects of other telomeric P elements – a phenomenon known as presetting (Niemi et al., Reference Niemi, Raymond, Patrek and Simmons2004) or as the pre-P cytotype (Ronsseray et al., Reference Ronsseray, Lemaitre and Coen1993).
At the molecular level, the synergism between telomeric and Birm P elements seems to involve a profound reduction in the amounts of P mRNAs. Our RT-PCR experiments indicate that the amount of a specific P mRNA was reduced in females that carried a telomeric P element, and reduced still further in females that also carried a set of the Birm P elements. Ping-pong cycling between the telomeric and Birm P elements therefore appears to eliminate P mRNA effectively.
When maternally transmitted, mutations in three genes – aub, piwi and Su(var)205 – interfered with the establishment of synergism between the telomeric P elements and the Birm P elements, and mutations in two of these genes – piwi and Su(var)205 – disrupted synergism that had already been established. All these mutations had previously been shown to impair some aspect of cytotype regulation (Ronsseray et al., Reference Ronsseray, Lehmann, Nouaud and Anxolabéhère1996; Marin et al., Reference Marin, Lehmann, Nouaud, Izaabel, Anxolabéhère and Ronsseray2000; Reiss et al., Reference Reiss, Josse, Anxolabéhère and Ronsseray2004; Haley et al., Reference Haley, Stuart, Raymond, Niemi and Simmons2005; Simmons et al., Reference Simmons, Ryzek, Lamour, Goodman, Kummer and Merriman2007b; Josse et al., Reference Josse, Teysset, Toideschini, Sidor, Anxolabéhère and Ronsseray2007). Our analysis of the synergistic aspect of this regulation was limited to the heterozygous effects of these mutations. That such effects were detected indicates that P-element regulation is impaired by simply depleting the proteins encoded by the aub, piwi and Su(var)205 genes. These proteins are therefore needed in quantity to establish and/or maintain the P cytotype.
The Aub protein, a member of the Piwi class of proteins, has been implicated in the generation, transport or targeting of antisense piRNAs and is thought to play an important role in the ping-pong cycle (Brennecke et al., Reference Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam and Hannon2007, Reference Brennecke, Malone, Aravin, Sachidanandam, Stark and Hannon2008). Genetic data indicate that Aub is critical for the establishment of P-element regulation in the female germ line (Simmons et al., Reference Simmons, Niemi, Ryzek, Lamour, Goodman, Kraszkiewicz and Wolff2007a). This regulation is impaired when aub mutations are crossed into females that contain only telomeric P elements, but not when they are crossed into females from P strains such as Harwich (Reiss et al., Reference Reiss, Josse, Anxolabéhère and Ronsseray2004). The insensitivity of the latter females to mutational depletion of the Aub protein could be due to the fact that ping-pong cycling has already generated a large pool of P-specific piRNAs in them. Females from TP; Birm strains would also be expected to be insensitive to Aub depletion – an observation reported here – because, like females from P strains, they would have benefited from ping-pong amplification of the P-specific piRNA pool. By contrast, females that contain only telomeric P elements would not have benefited from this amplification. Depletion of Aub in these females would therefore be expected to stymie the production of P-specific piRNAs and handicap the ability of these females to repress hybrid dysgenesis in their offspring.
The Piwi protein, like Aub, is involved in the piRNA pathway (Brennecke et al., Reference Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam and Hannon2007). It may also play a role in organizing chromatin within the TAS (Yin & Lin, Reference Yin and Lin2007). Heterozygous piwi mutations do not impair TP-mediated repression of P-element excisions in males (Simmons et al., Reference Simmons, Ryzek, Lamour, Goodman, Kummer and Merriman2007b). However, by testing for repression of GD in females we found that one of the piwi mutations (piwi1) impaired the establishment of synergistic regulation between one of the telomeric P elements (TP5) and the Birm P elements. This same mutation also disrupted synergistic regulation when it was crossed into a TP5; Birm strain. It is not clear why neither piwi1 nor piwi2 had any effect on synergistic regulation involving TP6. One possibility is that the Piwi protein regulates expression of the piRNA locus in the TAS of XL by binding to it (see Yin & Lin, Reference Yin and Lin2007), and this regulation is influenced by the overall structure of the XL telomere, which is likely to be different in the TP5 and TP6 X chromosomes (Thorp et al., Reference Thorp, Chapman and Simmons2009). It is also not clear why the piwi2 mutation did not affect TP-Birm P synergism. Like piwi1, this mutation is due to the insertion of a P transgene into the piwi gene. However, in piwi2 the insertion is in a coding region within exon 4 whereas in piwi1, it is in a non-coding region within exon 1 (Cox et al., Reference Cox, Chao, Baker, Chang, Qiao and Lin1998). Both alleles cause homozygous females to be sterile, but only piwi1 causes homozygous males to be sterile (Lin & Spradling, Reference Lin and Spradling1997). These observations suggest that the N-terminal polypeptide that might be produced by piwi2 is partially functional, thereby allowing the P cytotype to be established and maintained in TP-Birm combinations.
The Su(var)205 gene encodes heterochromatin protein 1 (HP1, also denoted HP1a), a protein that plays an important role in chromatin organization. HP1 is located in the centric heterochromatin, at the telomeres where it appears to cap the ends of chromosomes, and at scattered sites in the euchromatin (James et al., Reference James, Eissenberg, Craig, Diethrich, Hobson and Elgin1989; Fanti et al., Reference Fanti, Giovinazzo, Berloco and Pimpinelli1998; Perrini et al., Reference Perrini, Piacentini, Fanti, Altieri, Chichiarelli, Berloco, Turano, Ferraro and Pimpinelli2004). Brower-Toland et al. (Reference Brower-Toland, Findley, Jiang, Liu, Yin, Dus, Zhou, Elgin and Lin2007) have shown that it physically interacts with Piwi, and that HP1 and Piwi co-localize to many sites in the polytene chromosomes of larvae, including at the telomeres. These authors suggest that the ability of Piwi to interact with HP1 may facilitate the recruitment of HP1 to specific regions of chromatin. However, the ways in which Piwi and HP1 affect chromatin organization – possibly in collaboration with guiding piRNA molecules – may be complex (Brower-Toland et al., Reference Brower-Toland, Findley, Jiang, Liu, Yin, Dus, Zhou, Elgin and Lin2007; Yin & Lin, Reference Yin and Lin2007).
Ronsseray et al. (Reference Ronsseray, Lehmann, Nouaud and Anxolabéhère1996) showed that cytotype regulation is drastically impaired when a Su(var)205 mutation is crossed into TP strains; less impairment is seen when the mutation is crossed into P strains. Heterozygous Su(var)205 mutations also curtail the telomere trans silencing effect (TSE), a phenomenon that closely parallels cytotype regulation (Josse et al., Reference Josse, Teysset, Toideschini, Sidor, Anxolabéhère and Ronsseray2007); moreover, this curtailment is exacerbated in flies that are also heterozygous for a piwi mutation. We found that females from TP stocks that were heterozygous for a Su(var)205 mutation had little or no ability to establish synergistic regulation with the Birm P elements, and that already established synergism was partially disrupted when a Su(var)205 mutation was crossed into a TP; Birm stock. All these observations indicate that HP1 plays important roles in the processes involved in cytotype regulation. One possibility is that HP1, in collaboration with Piwi, regulates the expression of telomeric piRNA loci (Yin & Lin, Reference Yin and Lin2007). This regulatory role is consistent with the epigenetic aspects of the telomere TSE analysed by Josse et al. (Reference Josse, Teysset, Toideschini, Sidor, Anxolabéhère and Ronsseray2007). Another possibility is that HP1 regulates the size of the retrotransposon array at the ends of chromosomes. Depletion of HP1 causes these arrays to grow, creating elongated telomeres (Savitsky et al., Reference Savitsky, Kravchuk, Melnikova and Gvozdev2002). These genetic – rather than epigenetic – changes would be expected to affect the expression of piRNA loci situated proximally in the TAS. This possibility is consistent with the observation of Haley et al. (Reference Haley, Stuart, Raymond, Niemi and Simmons2005) that cytotype regulation is impaired many generations after a Su(var)205 mutation was removed from a TP stock.
Cytotype regulation was once thought to be mediated by P-encoded polypeptides (Rio, Reference Rio1990). Subsequent analyses have demonstrated this hypothesis to be inadequate (Stuart et al., Reference Stuart, Haley, Swedzinski, Lockner, Kocian and Simmons2002; Josse et al., Reference Josse, Teysset, Toideschini, Sidor, Anxolabéhère and Ronsseray2007; Jensen et al., Reference Jensen, Stuart, Goodpaster, Goodman and Simmons2008). Some form of regulation involving P-specific piRNAs and the proteins encoded by several genes, including aub, piwi and Su(var)205, now appears to be the mechanistic basis of the P cytotype.
C. B. benefited from a sabbatical furlough from Normandale Community College. This work was supported by funds from the Department of Genetics, Cell Biology and Development of the University of Minnesota. Johng K. Lim and two anonymous reviewers kindly provided many helpful suggestions to improve the original manuscript.











