Streptococcus pneumoniae is one of the major pathogens of pneumonia, otitis media, sinusitis, septicaemia, and meningitis in children. The World Health Organization (WHO) estimated that 1·6 million people died of pneumococcal disease in 2005, including 0·7–1 million children (aged <5 years) mostly from developing countries [Reference World1]. Moreover, multidrug-resistant S. pneumoniae isolates have markedly increased especially in Asia, and these strains continue to be a major cause of morbidity and mortality.
More than 92 serotypes of S. pneumoniae are recognized by the Quellung reaction. One of the most common is serotype 19F, which caused disease in many countries before the introduction of the pneumococcal conjugate vaccine [Reference Song2] and is also an important serotype of multidrug-resistant S. pneumoniae. Taiwan19F-14, a widespread resistant clone in Asian countries, was first identified in Taiwanese hospitals in 1993 [Reference Shi3], before it was disseminated to other countries. This clone was determined as sequence type 236 (ST236) by multi-locus sequence typing (MLST), and belongs to the epidemic clonal complex 271 (CC271). The spread of CC271 isolates has been implicated in the rapid increase of penicillin- and macrolide-resistant S. pneumoniae in Asia and the USA [Reference Gertz4].
A study of Chinese children hospitalized for pneumonia revealed that serotype 19F was responsible for half of all penicillin-resistant isolates [Reference Yao5], but to date such isolates have not been subjected to molecular epidemiological studies in China. In the present study, we describe the genotype distribution of serotype 19F S. pneumoniae and its association with antibiotic susceptibilities, including the temporal trend from 1997 to 2010 in Beijing, China.
The study protocol was in accordance with the ethical standards of the responsible regional committee on human experimentation and the Declaration of Helsinki 1975 (revised 1983); it was approved by the ethics committee of the Beijing Children's Hospital. Nasopharyngeal swabs were taken with written informed consent from the parents or legal guardians of the children. Data on the clinical isolates, methods of antimicrobial susceptibility testing, macrolide-resistance gene detection, MLST, and pulsed-field gel electrophoresis (PFGE) have been described in our previous report [Reference Ma6]. The antibiotics tested included penicillin (PEN), amoxicillin-clavulanic acid (AMC), ceftriaxone (CRO), cefuroxime (CXM), cefaclor (CEC), erythromycin (ERY), vancomycin (VAN), imipenem (IPM), levofloxacin (LVX), tetracycline (TCY), sulfamethoxazole-trimethoprim (SXT), and chloramphenicol (CHL).
Serotype 19F S. pneumoniae was identified in 11·5% (130/1132) of isolates during the entire study period. Their frequencies for different years were 6.1% (1997–1998), 9.4% (1999–2000), 9.5% (2001–2002), 11.2% (2003–2004), 13.1% (2005–2006), and 17.8% (2010). The percentage of serotype 19F isolates increased year by year, and become the most common serotype in 2010. Following the universal administration of the heptavalent pneumococcal conjugate vaccine (PCV-7), a significant decrease of serotype 19F was reported in the USA [Reference Hicks7], Korea [Reference Song8], and other countries. In China, PCV-7 was introduced in September 2008 in the private sector. However, this vaccine has not been included in the national immunization programme and the vaccination rate is very low. Thus, the frequency of serotype 19F did not decrease in 2010, and even increased.
All 120 available serotype 19F strains were susceptible to VAN and LVE (Supplementary Table S1, available online). The resistance rate against β-lactam antibiotics showed an increasing temporal trend. From 1997 to 2010, the non-susceptibility rates to PEN and AMC slightly increased, whereas the non-susceptibility rates to CEC and CXM significantly increased from 14·2% in 1997–1998 to more than 80% in 2010. By contrast, the non-susceptibility rate to CHL decreased from 28·5% in 1997–1998 to 11·1% in 2005–2006 and finally to zero in 2010. Most (99·2%) of the strains were non-susceptible to ERY, and 96·6% had high minimum inhibitory concentrations (MICs) (>256 μg/ml). The non-susceptibility rates to TCY and SXT exceeded 95% with almost all strains showing co-resistance to ERY, TCY, and SXT.
Of the 119 ERY non-susceptible pneumococci, ermB and mefA were detected in 115 (96·6%) and 64 (53·8%) isolates, respectively. Both ermB and mefA were detected in 60 (50·4%) strains.
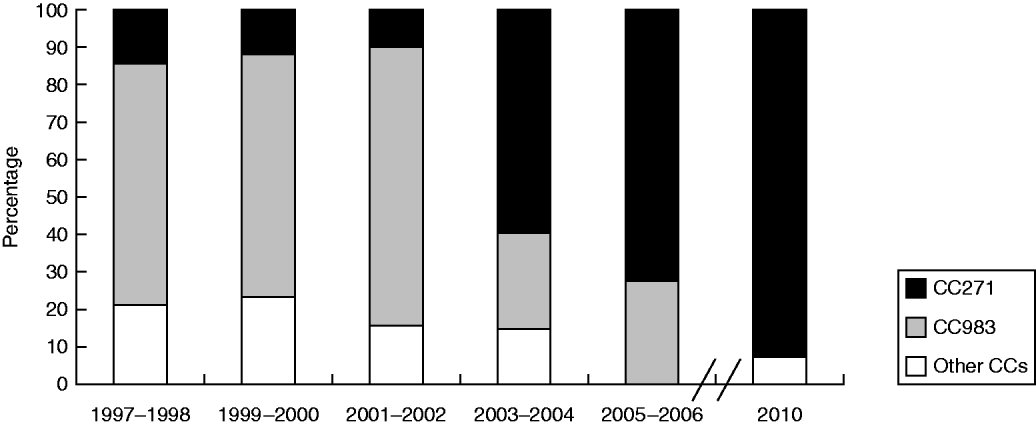
MLST analysis of the 120 serotype 19F isolates identified 31 STs, of which 13 STs were novel (ST8214–8227). The most common were ST983 (39/120, 32·5%), ST271 (13/120, 10·8%), ST6993 (7/120, 5%), and ST236 (6/120, 5%). eBURST analysis revealed the presence of five CCs and five singletons (Supplementary Fig. S1, available online). CC271 was the most common group comprising 14 STs and accounted for 48·3% of all strains while CC983, with five STs, accounted for 38·3% of the studied strains. Figure 1 shows that the distribution of the predominant CCs in serotype 19F strains varied during the study period. From 1997 to 2010, the frequency of CC271 increased from 14·3% in 1997–1998 to 72·2% in 2005–2006, and reached 92% in 2010, whereas CC983 decreased from 64·3% to 27·7% and finally to zero in the corresponding periods. The genetic homology of the strains was confirmed by PFGE, which revealed seven different patterns (A–G). CC271 was mainly associated with patterns C, D, E, and F, whereas CC983 was associated only with pattern F (Supplementary Fig. S2, available online).

Fig. 1. Distribution of the predominant clonal complexes (CCs) in serotype 19F S. pneumoniae strains during the study period.
CC271 was the predominant CC from 2003 to 2010 and was associated with multiple resistance and carriage of the ermB and mefA genes. The high frequency of CC271 has been reported as a major reason for antimicrobial resistance in S. pneumoniae isolates in Korea [Reference Song8], USA [Reference Bowers9], and Finland [Reference Siira10]. This clone could spread into the hinterland of China because of the increased international movements of populations.
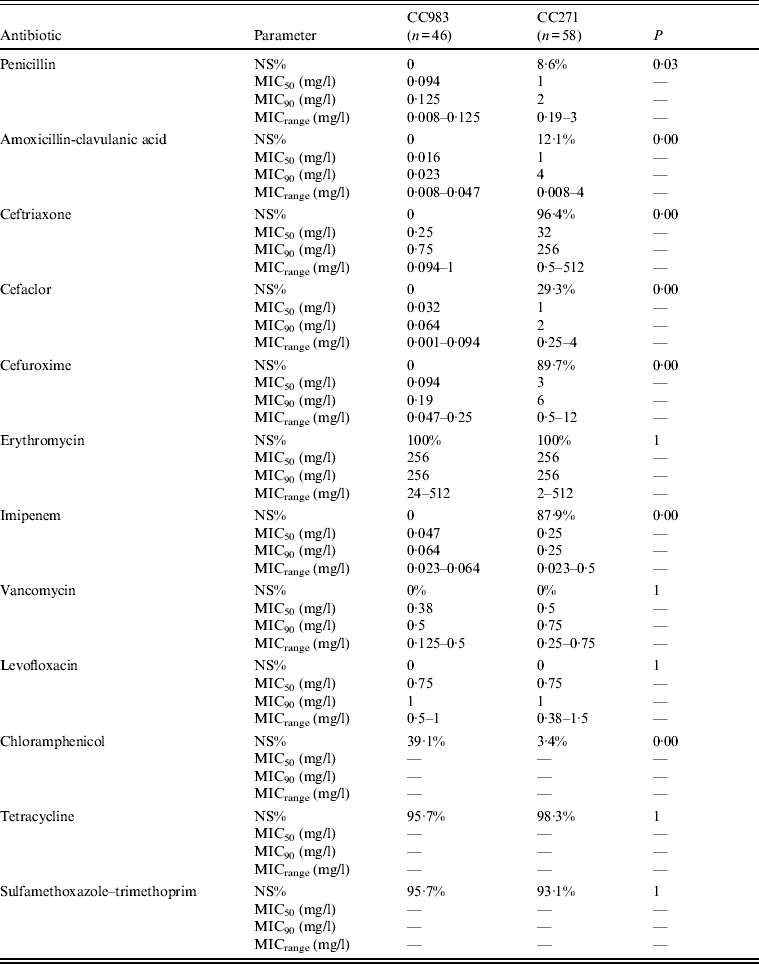
Significant differences between CC271 and CC983 in terms of antimicrobial non-susceptibility were found in this study (Table 1). All CC983 strains were susceptible to β-lactam antibiotics while extensive β-lactam resistance was a hallmark of most CC271 strains characterized by higher MIC50 and MIC90 values for these antibiotics than CC983. By contrast, CC983 were significantly more resistant to CHL than CC271. Both clones exhibited similar resistance to ERY, TCR, and SXT but most CC271 strains carried both macrolide resistance, i.e. ermB and mefA (96·6%, 56/58), while most CC983 strains were positive for ermB alone (91·3%, 42/46) and only three strains harboured both ermB and mefA (18·7%, 3/46).
Table 1. Difference between the antimicrobial non-susceptible profiles of CC271 and CC983 of serotype 19F S. pneumoniae

NS%, Percentage of non-susceptibility; MIC50, 50% minimum inhibitory concentration; MIC90, 90% minimum inhibitory concentration; MICrange, range of minimum inhibitory concentration.
The results of the study indicate a significant clonal shift of serotype 19F S. pneumoniae from CC983 to CC271 in Beijing from 1997 to 2010. Given the discrepancy of antibiotic resistance between the two CCs, the epidemic genotype replacement was deduced to be under antibiotic selective pressure. Antibiotic surveillance from 2002 to 2006 showed that the use of cephalosporins in Beijing Children's Hospital accounted for 35·0% of all prescribed antibiotics [Reference Zhang11]. The spread of the highly resistant CC271 may be driven by the selective pressure from antibiotic use, whereas the spread of the generally susceptible CC983 was limited.
In a previous study [Reference Yao12], we emphasized that overuse of β-lactam antibiotics might have created favourable conditions for the spread of serogroup 19 (especially 19A) and subtype 6B-II before the introduction of PCV-7 in China. The present results further support the view that the profound change in the S. pneumoniae populations in Beijing children may be a consequence of antibiotic overuse considering that genotype replacement should not be affected by vaccine immunization uptake.
The disappearance of CHL non-susceptible strains (28·5% in 1997–1998 to zero in 2010) was noted to be accompanied by the shift in the predominant clones. This decrease was most probably due to the limited administration of CHL in paediatric practice throughout the country since the 1990s, and markedly was not apparently associated with the loss of resistance by strains but with the elimination of the original resistant clones (CC983) by the use of other antibiotics. This indicates that resistance loss could not be verified after the antibiotic selective pressure was removed. At the same time, the absence of resistance of CC271 to CHL further suggests that this group had spread to Beijing from other regions at the beginning of this century.
An obvious limitation of the study is the fact that more hospitals were not surveyed for S. pneumoniae and that continuity of sampling was disrupted from 2007 to 2009. However, considering the large number of patients’ visits to the hospital (average of 5000–6000 visits per day) and the total pneumococcal isolates, we believe that the present results show that major epidemiological changes of serotype 19F S. pneumoniae have occurred in Beijing.
In summary, we have documented an increase in the prevalence of serotype 19F in S. pneumoniae isolates from children in Beijing, and also increased resistance to β-lactam antibiotics of this serotype. Clonal replacement under the selective pressure of β-antibiotics overuse is indicated by the spread of CC271 and the disappearance of CC983 and underlined by the fact that overuse of antibiotics has had an important effect on the population constitution of S. pneumoniae serotype 19F. Further long-term surveillance of this and other serotypes of pneumococci are necessary to monitor ST prevalence and associated antimicrobial resistance of this important human pathogen.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813000514.
ACKNOWLEDGEMENTS
This study was supported by the Special Fund for High-level Personnel Development of the Beijing Health System (2011-3-052) and the Beijing Nova Programme (2008B64). The authors express their gratitude to Wei Gao, Yan Li and Lin Yuan from Beijing Children's Hospital for their work in sample collection. We are grateful for the use of the MLST database, which is located at Imperial College London and funded by the Wellcome Trust.
DECLARATION OF INTEREST
None.