INTRODUCTION
Leptospirosis is a zoonosis of global significance which causes deaths in humans annually [1]. It is also an important cause of death in dogs, especially those that are unvaccinated, improperly vaccinated or those vaccinated with vaccines which did not contain the prevalent serovars found in that particular area [Reference Brown2]. Leptospires have been isolated from both clinically ill and apparently healthy animals from a variety of geographic locations, e.g. 78·6% of isolates in Mexico [Reference De Fritas3], 1·5% of stray dogs studied in Barbados [Reference Jones4], in clinically ill dogs in Illinois [Reference Nielsen5], the skin of an aged dog [Reference Anderson6], an aborted foetus in Argentina [Reference Rossetti7] as well as laboratory dogs [Reference Ruhl-Fehlert8]. The clinical signs in dogs vary from mild to severe and include fever, vomiting, anorexia and jaundice [Reference Faine9]. Leptospira serovars Bratislava and Canicola tend to cause signs of renal dysfunction, whereas infections with Icterohaemorrhagiae and Pomona have been reported to produce hepatic damage [Reference Ananda10].
Almost every known species of rodent, marsupial and mammal can be a carrier and excretor of leptospires [Reference Faine9]. While there are characteristic associations of particular serovars with certain species of animals, the molecular basis for maintenance of host specificity is unknown [Reference Adler and de la Pena Moctezuma11]. Of the aforementioned, rodents appear to be the most important reservoirs of infection, contaminating the environment as well as food and transmitting their infection when consumed by carnivores [Reference Faine9]. Rats in particular are major sources of infection for humans and dogs. It has been speculated that the serovars present in rodents in a given environment are similar to those present in dogs living in that same environment. In addition, it has been found that very often the serogroups associated with rodents include Icterohaemorrhagiae [Reference Vijayachari, Sugunan and Shriram12] and Ballum [Reference Vanasco13].
To date, there is no consensus on the issue of cross-protection between serovars of Leptospira in vaccinated animals and it has been recommended that vaccines used should contain those serovars of Leptospira that are actually causing the disease in that particular geographic location [Reference Levett14]. It is therefore of epidemiological and disease-prevention relevance to determine the prevalent serovars in different geographic locations [Reference Okewole and Ayoola15] as the findings will have implications on policy such as rodent control, farm sanitation, waste disposal and vaccination protocol.
In Trinidad, the last published information on the isolation of Leptospira from both animals and humans was between 1976 and 1979 [Reference Everard16, Reference Everard and Green17], when 31 isolates were recovered from mongoose (five isolates, all of which were serovar Canicola), rodents (eight isolates of which one was Hebdomadis, four were Icterohaemorrhagiae, and three were Ballum) and humans (a total of 18 consisting of, two Grippotyphosa, one Tabaquite, three Trinidad, three Mini Georgia, three Canicola, two Icterohaemorrhagiae, one Hebdomadis, one Brasiliensis, one Tarassovi and one Pyrogenes). However, a recent serological study in 2006 detected leptospirosis in 14% of dogs sampled comprising a seroprevalence of 48% in suspected canine cases of leptospirosis, 25·5% in hunting dogs, 20·4% in farm dogs, 6·3% in apparently healthy dogs and 4·4% in stray dogs [Reference Adesiyun18]. In that study, serovar Mankarso was reported to be the predominant serovar and more importantly, vaccinated dogs were observed to exhibit clinical leptospirosis. The authors also found that immunoglobulins to the two serovars of Leptospira (Canicola and Icterohaemorrhagiae) in the vaccines used locally were rarely detected in seropositive animals, a finding which suggested that the vaccine serovars were unimportant in causing canine leptospirosis in Trinidad and that cross-protection against other serovars of Leptospira was minimal or not as significant as had been earlier reported [Reference Tabata19].
In order to adequately investigate leptospirosis in Trinidad, an insight into the occurrence of locally circulating serovars of Leptospira is required. The objectives of this study were thus to determine the frequency of isolation of Leptospira from dogs (owned suspected cases of leptospirosis and stray dogs) and captured wild rodents; to determine and compare the various serovars isolated from dogs and rodents in the country, and finally, to determine the predominant clinical and pathological (gross and histopathological) lesions in suspected clinical signs of canine leptospirosis.
MATERIALS AND METHODS
Types and sources of animals studied
In dogs and rodents, unclotted blood and kidneys were cultured for the isolation of leptospires.
Dogs
Suspected canine cases (n=50) were animals presenting to veterinary clinics across the country with clinical signs consistent with those displayed by animals with leptospirosis (based on the clinician's judgement, e.g. jaundice, anorexia and vomiting). Dogs were first examined and the data collected by the completion of a questionnaire for each dog. Blood was collected and some dogs were then euthanized, with the approval of the owner, and necropsies were performed on these dogs. Kidneys were removed and stored on ice until arrival at the laboratory.
Stray dog samples (n=207) were acquired through weekly visits to the Trinidad and Tobago Society for the Prevention of Cruelty to Animals (T&TSPCA). This is a central point where stray dogs collected from the streets by the Regional Health Authorities (RHA) from across the country were brought for euthanasia or to find homes for them. At the T&TSPCA, by law dogs are usually kept for 1 week in order to give owners an opportunity to claim them. All unclaimed dogs after the 7-day period were euthanized.
Wild rodents
Wild rats (Rattus spp.) (n=211) were trapped in an opportunistic manner using specially designed wire cages containing food baits. Once trapped, the cages containing the rodents were placed in ventilated black bags to reduce excitement and stress to the animals during transportation to the laboratory. The RHA and rodent control gangs across the country assisted in trapping rodents around homes, public buildings and food markets.
Administration of questionnaires to the owners of dogs
Questionnaires were administered to the owners of the suspected canine cases of leptospirosis to obtain epidemiological information such as the age, sex, breed, vaccination status, contact with rodents and other information about the progression, duration, clinical signs and treatment of the disease.
Collection of samples
In dogs, ~5 ml of blood was collected via venepuncture (using a 20G 1-inch needle, 5 ml syringe) of the cephalic vein. The blood samples were collected in heparinized tubes after which a few drops were inoculated directly into Ellinghauser–McCullough–Johnson Modified Harris (EMJH) semi-solid medium as recommended [Reference Faine9]. The remaining blood was sent to the Haematology Laboratory of the School of Veterinary Medicine for a complete blood count.
Clinical assessment of owned dogs with suspected leptospirosis
Clinical assessment of dogs was conducted by the veterinarian to whom the dog was presented and was based solely on clinical signs such as jaundice, anorexia and vomiting.
Euthanasia of dogs and kidney collection
For stray dogs as well as those suspected cases where the owners agreed to euthanasia, the dogs were euthanized by intravenous injection of 10% phenobarbital solution (Merial Animal Health, UK). After death, the kidneys were accessed via an incision in the left paralumbar fossa and removed, placed in sterile bags and then onto a cooler with ice packs at 4°C.
Euthanasia of wild rodents
Rodents were euthanized by first rendering them unconscious in a chamber which was slowly filled with carbon dioxide from a pressurized tank. When unconscious (detected by lateral recumbency and loss of pedal reflex), the rodents were anaesthetized using a combination of a 10% Ketamine solution (Dutch Farm Veterinary Pharmaceutical Company, The Netherlands) and Xylazine sold as Bromazine 2% solution (Bomac Laboratories, New Zealand). The minimal dosage given intramuscularly was 85 mg Ketamine mixed with 15 mg Xylazine/kg [Reference Wixson20]. If the desired response was not achieved, more of the mixture was given until there was no response to pain and the loss of righting reflex were observed. The thoracic cavity was then exposed and blood was collected by cardiac puncture with a 21G 1½-inch needle attached to a sterile 3 ml syringe (as approved by the Ethics Committee of the University of the West Indies and following the guidelines of the Canadian Council on Animal Care).
Necropsy of suspected canine cases of leptospirosis
Gross examinations of the carcasses were performed and these observations were recorded as digital images. Samples of the kidneys, livers, hearts, spleens and lungs were collected and stored in 10% buffered saline until they were processed for histopathological studies. Processed slides were stained with haematoxylin–eosin (H&E) prior to microscopic examination.
Culture and typing of Leptospira spp
For this study two forms of media were used, namely semi-solid and liquid EMJH media which were prepared as recommended by the Barbados Leptospira Laboratory, Barbados. The semi-solid medium was used for the isolation and maintenance of cultures and the liquid EMJH for the growth and maintenance of Leptospira isolates as antigens for the microscopic agglutination test (MAT) and isolates for typing by the serological methods used in this study. PLM5 (prepared Leptospira medium) was prepared using the method prescribed by the manufacturer.
Culture of Leptospira spp. from blood
For each blood sample, two drops (~100 μl) were inoculated into a tube containing 4·0 ml EMJH semi-solid medium and incubated at 28–30°C. At weekly intervals for up to 3 months, the samples were checked, using a dark-field microscope, for evidence of leptospires. All samples that failed to show any evidence of growth at 3 months post-inoculation were considered negative for leptospires.
Culture of Leptospira spp. from the kidneys
Kidney samples were placed into sterile Petri dishes and with the use of a sterile scalpel blade cut into 1-g wedges containing both the cortex and medulla and placed into three sterile Eppendorf tubes. In one of the tubes, a small plastic pestle was used to macerate the kidneys while the remaining two portions were stored, frozen at −80°C for PCR assay. To the macerated or homogenized kidney tissues, 900 μl of liquid EMJH medium was added. Thereafter, 100 μl of this solution was inoculated into a culture tube and a further 100 μl of this mixture was added to 900 μl of liquid medium in another Eppendorf tube and mixed using a vortex mixer. The suspension (100 μl) was used to inoculate the semi-solid medium. The inoculated semi-solid medium tubes were incubated at 28–30°C and checked each week for up to 3 months using dark-field microscopy. Samples were only classified as negative if leptospires were not detected in the culture 3 months post-incubation.
Typing of Leptospira isolates
Rabbit antisera
The 23 serovars of lyophilized antisera were purchased from the Koninklijk Instituut voor de Tropen/Royal Tropical Institute (KIT), Biomedical Research Laboratory, Amsterdam, The Netherlands. The international panel utilized consisted of the following serovars: Australis Bratislava, Autumnalis Bim, Autumnalis Autumnalis, Ballum Arborea, Ballum Ballum, Bataviae Bataviae, Canicola Canicola, Cynopteri Cynopteri, Grippotyphosa Grippotyphosa, Hebdomadis Hebdomadis, Icterohaemorrhagiae Copenhagani, Icterohaemorrhagiae Icterohaemorrhagiae, Icterohaemorrhagiae Mankarso, Mini Georgia, Panama Panama, Pomona Kennewicki, Pomona Pomona, Pyrogenes Pyrogenes, Sejroe Hardjo, Sejroe Sejroe, Sejroe Wolfii, Semaranga Patoc, Tarassovi Tarassovi. For each lyophilized antiserum in glass vials stored at −20°C, the quantity of sterile water recommended by the manufacturer was added for reconstitution and then shaken gently and used immediately. The remaining portions were stored at −20°C. The leptospires were counted using a dark-field microscope on the same day the titration was conducted. The isolates of leptospires were serotyped using standard procedures [21].
Monoclonal antibody testing
A panel of six monoclonal antibodies 12C3, 52C1, 70C7, 70C14, 70C20 and 89C12 recommended by the KIT, Royal Tropical Institute, Amsterdam, The Netherlands, was used to differentiate between serovars Copenhagani, Icterohaemorrhagiae and Mankarso. These six monoclonal antibodies were used in a previous study [Reference Korver22] to classify serovars of the Icterohaemorrhagiae serogroup using monoclonal antibodies.
This test was conducted on all the isolates which showed multiple agglutinations to rabbit sera of the Icterohaemorrhagiae group to determine the specific serovars to which they belonged. The methodology used was similar to that performed for the rabbit antisera described except that 10 μl of the reconstituted monoclonal antibody were added to the microtitre plate instead of rabbit antiserum.
Statistical analyses
All statistical analyses were conducted using SPSS software, version 15 (SPSS Inc., USA). The data were subjected to an independent-samples t test and the P values were generated. χ2 analysis was also performed using SPSS on this data and the P values seen in Table 1 were obtained. For the study, the confidence level was set at 95%.
Ethical approval
Prior to the commencement of the study, the Ethics Committee of the Faculty of Medical Sciences, University of the West Indies, St Augustine Campus, Trinidad approved the study after reviewing the research protocol.
RESULTS
Isolation of Leptospira spp. from dogs and rodents
The frequency of isolation of Leptospira spp. was 18·0% from suspected canine cases, 3·4% from stray dogs and 25·6% from rodents. Of the 468 animal samples subjected to culture 70 (15·0%) were culture-positive. Suspected cases of canine leptospirosis, 18·0% (9/50) had a significantly (P<0·05, χ2) higher frequency of isolation than stray dogs, 3·4% (7/207). The frequency of recovery of Leptospira, 25·6% (54/211) in rodents was, however, significantly (P<0·05, χ2) higher than that found in all dogs, 6·2% (16/257). This is due to the fact that rodents are reservoirs of leptospiral infection.
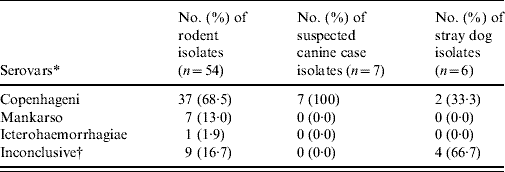
The relationship between isolation of leptospires and the clinical manifestations displayed by suspected cases of canine leptospirosis are displayed in Table 1. The frequency of clinical signs was not statistically significant (P>0·05, χ2) between the dogs that were negative or positive for the isolation of leptospires.
Table 1. Clinical signs observed in suspected canine cases (n=50)
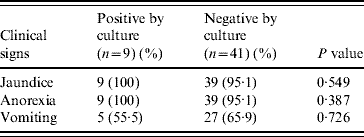
Table 2 shows the relationship of selected factors, specifically, vaccination status, exposure to rodents, sex, age and use for hunting. Age of the dog was the only risk factor that was statistically significantly (P=0·023) associated with the isolation of leptospires.
Table 2. Risk factors associated with leptospiral infection
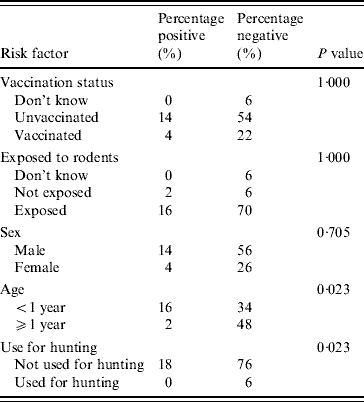
The identification of the isolates of leptospires found in dogs and rodents
With the use of an international panel of 23 rabbit antisera, in the typable isolates, the predominant agglutinations were to serovar Copenhageni, 71·4% (5/7) in suspected canine leptospirosis cases (Table 3). Multiple agglutinations with antisera, other than that of the Icterohaemorrhagiae serogroup, were noted in 33·3% (2/6) of stray dogs.
Table 3. The identities of isolates obtained by rabbit antisera technique
* Two isolates from suspect canine cases and one isolate from stray dogs were lost before serotyping was done.
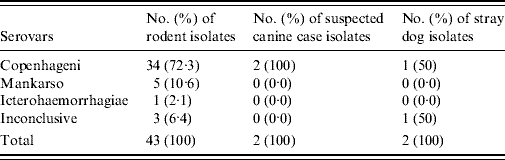
Monoclonal antibodies used to serotype the isolates of Leptospira spp. identified serovar Copenhageni as the predominant serovar in isolates from suspected canine leptospirosis cases, 100·0% (2/2), stray dogs, 50·0% (1/2) and rodents, 72·3% (34/43) as shown in Table 4.
Table 4. The identities of isolates obtained using the monoclonal antibody technique
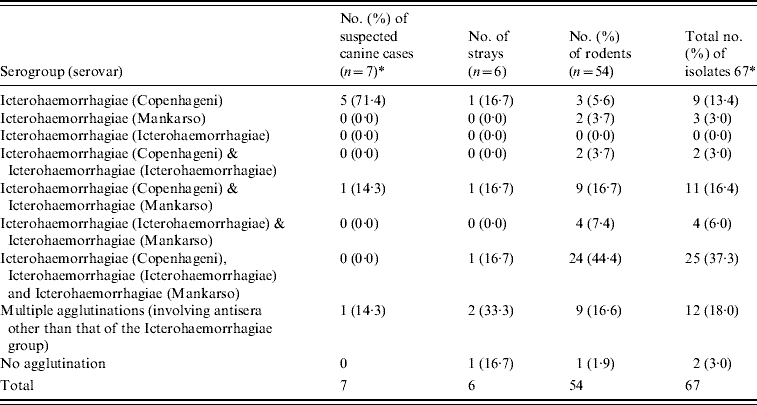
A combination of both rabbit antisera of the international panel and the six selected monoclonal antibodies for the Icterohaemorrhagiae group revealed that in all typable isolates, serovar Copenhageni was again most commonly detected with a frequency of 100% (7/7), 33·3% (2/6) and 68·5% (37/54) in suspected cases of canine leptospirosis, stray dogs and rodents, respectively (Table 5). The serotyping results were inconclusive for 13 isolates of Leptospira spp. tested.
Table 5. Summary of the number of isolates belonging to each serovar
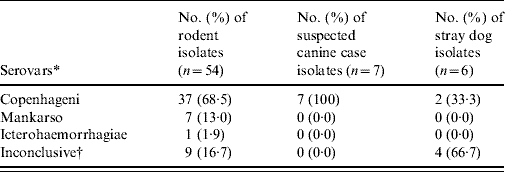
* Using the 23 international panel of sera and the panel of 6 monoclonal antibodies.
† Thirteen inconclusive after conducting serological tests.
The distribution of the serovars of Leptospira obtained by culture
Figure 1 shows the distribution of the serovars obtained by culture from suspected canine cases of leptospirosis. There appears to be a clustering of culture-positive samples in the county of Caroni. No map could be constructed for stray dogs as accurate data on the exact locations of these dogs were not available.
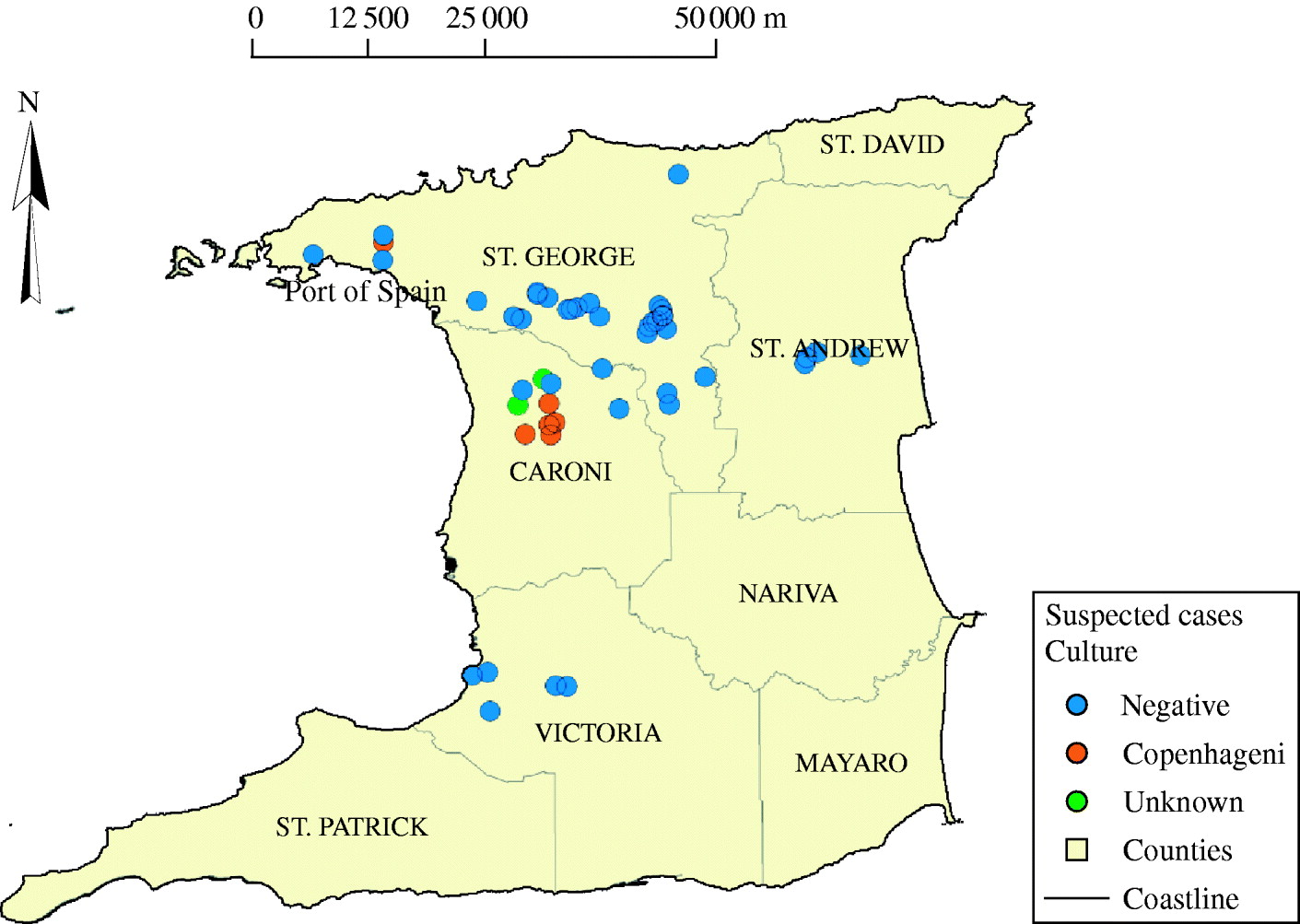
Fig. 1. Map of Trinidad showing the locations from which the positive and negative suspected canine cases were obtained.
Figure 2 shows the locations where the rodent samples were trapped and the corresponding serovars. Again, there is no definite pattern of distribution of positive samples or serovars.
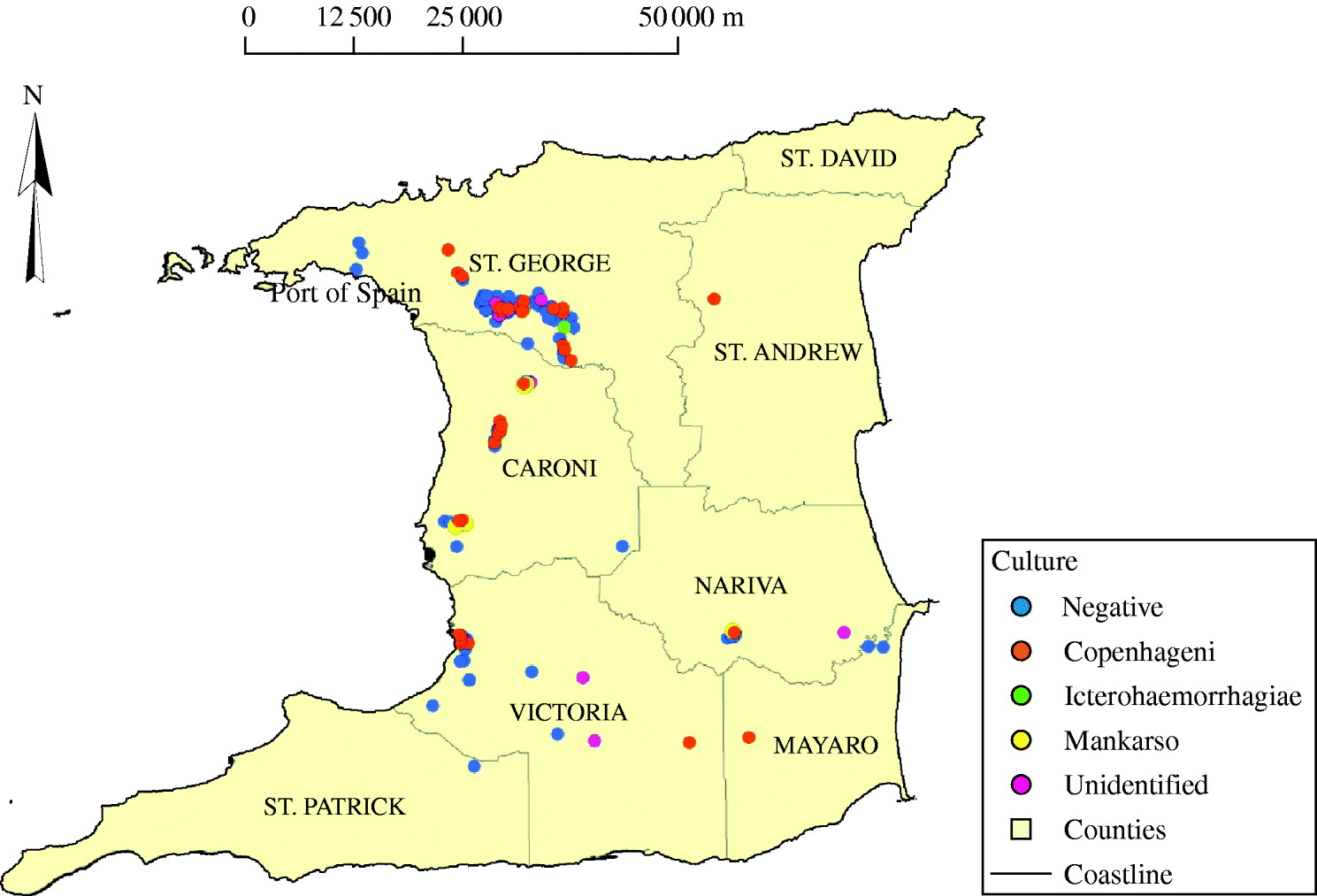
Fig. 2. Map of Trinidad showing the locations from which positive and negative rodents were obtained.
The pathological findings of Leptospira-positive dogs
Figure 3 displays representative gross pathological findings detected in Leptospira culture-positive, suspected, canine leptospirosis cases. Jaundice of the subcutaneous fat (Fig. 3 a), follicular hyperplasia in an enlarged spleen (Fig. 3 b), pale foci and petechial haemorrhages on the serosal surface of the liver and stomach (Fig. 3 c) were observed. Haemorrhagic contents of the stomach (Fig. 3 d) and the kidneys (Fig. 3 e) were also gross pathological lesions seen.

Fig. 3. Gross pathological lesions observed in the suspected canine cases. (a) A suspected canine case of leptospirosis exhibiting jaundice. (b) Follicular hyperplasia of an enlarged spleen of a culture-positive dog. (c) Liver and stomach of a culture-positive suspected canine case of leptospirosis. (d) The haemorrhagic contents of the stomach of a culture-positive suspected canine case. (e) The haemorrhagic kidneys of a culture-positive suspected canine case of leptospirosis. A, Yellow subcutaneous fat of the dog, indicative of jaundice; B, white nodular regions, which are follicles of the spleen which have become enlarged; C, pale foci on the serosal surface of the liver; D, petechial haemorrhages on the serosal surface of the stomach; E, the haemorrhagic contents of the stomach as well as haemorrhages on the mucosal surface of the stomach; F, haemorrhages at the corticomedullary junction of the kidney.
Histopathological lesions, following H&E staining of sections from culture-positive dogs included disorganization of the normal cord-like structure of the hepatoctes of the liver and breakdown of the tubular structure of the kidney as seen in Figure 4.

Fig. 4. Microscopic changes observed in the suspected canine cases compared to normal healthy dogs. (a) A histological slide stained with haematoxylin-eosin (H&E) stain of the disorganization of the normal cord-like structure of the liver of a culture-positive suspected canine case of leptospirosis. (b) Liver of a healthy dog showing the normal cord-like structure of the kidneys. (c) A histological slide stained with H&E stain showing the breakdown of the normal tubular structure of the kidney in a culture-positive suspected canine cases. (d) Kidneys of a normal dog. G, Disorganization of cord-like structure and haemorrhage into extracellular space; H, destruction of the normal tubular structure.
DISCUSSION
Suspected canine cases
The results on the prevalence of leptospirosis by culture is 18·0% in suspected canine cases and all isolates were obtained from the culture of blood, except for one case in which both blood and kidney were positive. Epidemiological data collected on the suspected cases of canine leptospirosis demonstrated that the three major clinical signs (jaundice, anorexia and vomiting) occurred at similar frequencies in dogs positive or negative for the isolation of leptospires. This indicates that either these signs are not pathognomonic for leptospirosis or that the suspected cases of leptospirosis were wrongly diagnosed (i.e. false-positive) since only 18% of these dogs yielded leptospires. Reports by other studies [Reference Faine9, Reference Po-Chang23] have, however, demonstrated that these clinical signs are frequently associated with leptospirosis in clinically ill dogs.
This frequency is similar to the 20% isolation rate reported previously [Reference Everard16]. The isolates recovered belonged to serovar Copenhageni (7/7 isolates), an indication that this may be an important serovar responsible for canine leptospirosis in the country. Failure to detect any relationship between isolation of leptospires from suspected canine cases and the three clinical signs in the current study could not be due to the reported effect of the phase of infection in dogs on the frequency of isolation from the blood during the acute phase of the disease and the kidney during the chronic phase [Reference Faine9] because both kidney and blood from each dog were cultured for leptospires. The findings may be explained, in part, by the sensitivity of the culture method used in the study, reported to range from 89% to 93% [Reference Wagenaar24].
The widespread nature of leptospirosis is suggested by the fact that there was no definite pattern in the distribution of culture-positive dogs and the infecting serovars (Fig. 1). This may be reflective of environmental exposure of dogs, particularly to rodents, which had the highest prevalence of infection and carried similar serovars isolated from dogs. Rodents are known reservoirs for canine leptospirosis [Reference Vanasco13].
Pathogenic serovars of Leptospira have been known to cause different pathological changes, both gross and histological, in the tissues of infected animals [Reference Vanasco13, Reference Jones, Hunt and King25]. A number of these gross changes included jaundice and petechial haemorrhages distributed over various surfaces, swollen kidneys with subcapsular haemorrhages and enlarged, dark and soft spleens. Histopathological lesions such as loss of organized cord and lobular structure in the liver, interstitial nephritis and tubular necrosis in the kidney were observed in the current study.
Of interest was the fact that of the five risk factors for leptospirosis (age, sex, vaccination status, exposure to rodents and use as hunting dogs) reported to be associated with infection in dogs [Reference Meeyam26], only age was found to be statistically significantly associated with the isolation of leptospires from the suspected canine cases of leptospirosis. It has been previously established that the age of the dog is an important risk factor for leptospirosis and that older dogs are significantly more at risk, primarily based on serological findings reflective of exposure experience [1, Reference Faine9]. The finding in the current isolation study is that dogs aged <1 year were significantly more likely to be culture-positive than older dogs. This did not, however, come as a surprise. Their susceptibility to infection by leptospires is the basis for the recommended vaccination protocol in dogs in Trinidad starting at 6 weeks of age. The failure to detect any association between the use of dogs for hunting or sex of dogs and isolation of leptospires in the current study agrees with published reports [Reference Adesiyun18, Reference Ward27, Reference Ward, Glickman and Guptill28].
It was important to note that vaccination of dogs did not significantly affect the frequency of isolation of leptospires from these animals. This finding is in accord with a previous study where leptospirosis was detected in vaccinated dogs in Trinidad [Reference Adesiyun18] reported a similar finding. These findings, along with reports from local veterinarians of an increase in clinical canine leptospirosis in properly vaccinated dogs, suggest that vaccines used locally to protect against canine leptospirosis are not effective. This was not unexpected because the vaccines used locally are all killed and contain only two serovars, namely Canicola and Icterohaemorrhagiae. Both serovars have been demonstrated serologically [Reference Adesiyun18] and by culture in the current study to be unimportant in causing canine leptospirosis in Trinidad. On the other hand, serovars Mankarso and Copenhageni were found to be significantly responsible for canine leptospirosis in the country. It has been suggested that for dogs to be protected against leptospirosis, vaccines containing the prevalent serovars should be used [Reference Levett14].
Stray dogs
The frequency of isolation (3·4%) of Leptospira from the kidneys of stray dogs in the current study is low but comparable to the reports from other tropical and sub-tropical environments. In São Paulo, Brazil, an isolation rate of 2·4% 1415 dogs was reported [Reference Yasuda29] while in Barbados, a frequency of isolation of 1·5% was documented in 130 stray dogs tested [Reference Jones4]. The 3·4% frequency found in the current study is, however, considerably lower than the isolation rate of 20% in the last published study conducted 30 years ago in the country [Reference Everard16]. This may reflect a change in the level of infection by leptospirosis in the country over time. All the isolates from the stray dogs in this study were cultured from the kidneys and none from the blood sampled from this group of dogs was found to be culture positive. This, combined with the fact that no clinical signs consistent with leptospirosis were observed, suggest that these were not acute infections and it is likely that these dogs were previously infected, survived the infection to become renal carriers and thus persistent shedders [Reference Adler and de la Pena Moctezuma11].
Both isolates from stray dogs that were typable belonged to serovar Copenhageni as was similarly detected in stray dogs from Barbados; however, it is pertinent to mention that four (66·7%) of the six isolates of Leptospira spp. from stray dogs in the current study were untypable using the currently available panel of antisera. There is a need to explore ways to type these leptospiral isolates as they may be novel serovars or strains of Leptospira.
Rodents
A frequency of isolation of 25·6% for Leptospira spp. from rodent kidneys in our study is considerably higher than the range of 9·9% [Reference Cho30] in rodents in Korea to 19% in Barbados during 1986/1987 and 16% positive during 1994/1995. The predominant isolates were Copenhageni followed by Arborea followed by Bim [Reference Levett31]. Isolation rates as high as 82·9% in mice in the Azores [Reference Collares-Pereira32] have been reported. As expected, all rodent blood samples tested were negative for leptospires and all isolates were recovered from the kidneys, again reflecting the chronic nature of infection in rodent reservoirs [Reference Sunbul33]. That the predominant isolates were Copenhageni followed by Arborea and Bim is consistent with a previous study conducted in Barbados [Reference Levett31].
Again, in rodents, isolates of Leptospira serovar Copenhageni (68·5%) and Mankarso (13%) were the most frequently detected in the typable isolates, a finding consistent with the fact that serovar Copenhageni is often associated with infection in rodents. In the last published study on the isolation of Leptospira spp. conducted in Trinidad, in 1976 [Reference Everard and Green17]. The serogroups Hebdomadis, Icterohaemorrhagiae and Ballum were recovered but identification was not performed up to the serovar level to allow a comparison between that study [Reference Everard16] and our study.
Untypable Leptospira isolates
It is of diagnostic relevance that 19·4% (13/67) of all the isolates could not be conclusively identified, suggesting that these isolates may be novel serovars, or that they belong to serovars which are rare and hence not detected by the panel of international antisera used in this study. It is imperative that these isolates be subjected to further testing including PCR, followed by the multilocus sequence typing (MLST) [Reference Ahmed34] to determine their identities.
Implications of the presence of the serovars identified
The overwhelming occurrence of serovar Copenhageni from 85·2% (46/54) of the typable isolates, recovered from dogs and rodents studied has significant implications for the prevention of canine leptospirosis, more so, that all the vaccines available locally do not contain this serovar. In addition, serovar Mankarso which represented 13·0% (7/54) of the typable isolates is also not among the serovars in the vaccine. A similar phenomenon was observed in a study in Nigeria where the predominant serovars identified in dogs were Grippotyphosa, Pomona and Bratislava, which were non-vaccinal serovars [Reference Okewole and Ayoola15]. A vaccine containing these two serovars (Copenhageni and Mankarso) may therefore be more effective for use in Trinidad and Tobago.
It is clear that rodents are the maintenance hosts of serovar Copenhageni in Trinidad and possibly a significant source of this important serovar for canine infection. This emphasizes the importance of rodent control as one of the measures that may be used to control canine leptospirosis.
The apparent change in the predominant serovar from Mankarso detected in a serological study [Reference Adesiyun18] to Copenhageni in this study, conducted by isolation, may be due to reasons previously proposed [Reference Thaipadungpanit35]. The possibility that Copenhageni has developed a competitive advantage over Mankarso such as a selective advantage for Copenhageni in the maintenance host or when Copenhageni are shed into the environment from the hosts, they are able to resist detrimental environmental factors better than Mankarso. The current study has therefore established the need for a change in the vaccines used for the prevention of canine leptospirosis in Trinidad and Tobago. It is imperative that for any commercial vaccine to be effective in preventing canine leptospirosis locally, it should contain serovar Copenhageni and Mankarso.
ACKNOWLEDGEMENTS
We thank University of the West Indies School of Graduate studies and Research for funding. Veterinarians of participating clinics for their time and effort, Regional Corporations and students of the School of Veterinary Medicine for their cooperation in trapping rodents, Dr Ravi Seebaransingh for help with the histology of the samples and Sannandan Michael Samlal for technical support.
DECLARATION OF INTEREST
None.