Introduction
Until 2020, tuberculosis (TB) was the leading cause of death from a single infectious agent and claimed the lives of at least 1.4 million individuals in 2019 [1]. In sub-Saharan Africa, the treatment success rate in bacteriologically confirmed TB patients was recently reported to be only 76% against a global target of 90% [Reference Izudi2]. There is the need to identify and modify risk factors for poor treatment outcomes to realise the aims of the ‘End TB Strategy’ that espouses management of comorbidities as a key component of integrated patient care and prevention [Reference Uplekar3].
Anaemia is associated with a fourfold risk for TB infection and a dose-dependent relationship between anaemia severity and TB risk has been demonstrated in a recent systematic review and meta-analysis [Reference Gelaw, Getaneh and Melku4]. Further, anaemia is common among people with TB with an estimated prevalence of about 62%, and 36%, 31% and 12% of patients are reported to have mild, moderate and severe anaemia, respectively [Reference Barzegari5]. TB-associated anaemia has multiple causes, including suppression of erythropoiesis by inflammatory markers as well as nutritional deficiency [Reference Minchella6]. The high prevalence of anaemia in TB is concerning because of its association with delayed sputum conversion [Reference Nagu7], severe forms of TB (such as meningitis and disseminated disease) [Reference de Mendonça8], TB-related mortality and TB recurrence [Reference Isanaka9]. It is, therefore, imperative to screen for anaemia among people with TB and institute timely interventions.
Uganda is an HIV/TB high-burdened country which notified almost 66 000 TB cases in 2019 and registered a TB treatment success rate of 74% for the 2017 cohort [1]. Malnutrition accounts for a higher number of the estimated incident TB cases (42 000) than HIV infection (33 000) [1]. However, the burden of anaemia in TB and its associations are not well established in Uganda. A study conducted more than 20 years ago at the National Tuberculosis Treatment Center (NTTC) reported anaemia among 63% and 43% of men with TB with and without HIV co-infection, respectively, while 86% and 68% of women with TB with and without HIV co-infection had anaemia, respectively [Reference Shah10]. Additionally, TB was the commonest diagnosis (diagnosed among 22%) in people with anaemia at the emergency unit of a national referral hospital [Reference Mukaya11]. We have recently shown that HIV-negative TB patients with anaemia were more likely to have low CD4 T-lymphocytes at the NTTC [Reference Baluku12]. These studies suggest that the prevalence of anaemia among TB patients in Uganda may be high and could be associated with impaired immune responses and severe TB disease. The objective of this study was to determine the prevalence of anaemia and associated factors among people with bacteriologically confirmed TB at the NTTC in Uganda.
Materials and methods
Study design, population and setting
Using a cross-sectional study design, people presenting to the NTTC at Mulago National Referral Hospital (MNRH) in Uganda were enrolled consecutively between August 2017 and March 2018. The NTTC is an urban tertiary care facility for adult in-patient and outpatient TB care located in Kampala, the capital city of Uganda. It is a centre of excellence for TB care where both drug-sensitive and drug-resistant TB cases are managed. Less than 30% of TB cases managed at the facility are referrals from peripheral facilities. Eligible people were adults (≥18 years) who had pulmonary TB that was bacteriologically confirmed by sputum smear microscopy (Auramine staining), Xpert MTB/RIF assay and/or sputum mycobacterial culture (Löwenstein–Jensen medium). People who had received TB therapy for at least 2 weeks were excluded as TB therapy beyond 2 weeks alters clinical variables: mycobacterial burden, symptoms and haemoglobin levels [Reference Wilson13, Reference Finney14]. The findings of the primary study have been published elsewhere [Reference Baluku15]. In this secondary analysis, we included all people in the primary database who had haemoglobin measurement performed. MNRH is a tertiary health care facility located in Kampala, the capital city of Uganda.
Study definitions and measurements
Details of the study measurements are provided elsewhere [Reference Baluku15]. Briefly, a study questionnaire that sought for demographic data, medical history and symptoms was administered by trained research assistants. The participants' weight and height were measured using a weighing scale (Seca 760®) and stadiometer (Seca 213®), respectively, and the body mass index (BMI) was computed using the formula: BMI = (weight in kilograms)/(height in centimetres)2. BMI was graded as: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2) and overweight (≥25.0 kg/m2) [Reference Bailey and Ferro-Luzzi16]. An axillary temperature was measured using a digital thermometer and graded as: hypothermia (<35.5 °C), normal (35.5–37.4 °C) and hyperthermia (≥37.5 °C) [Reference Sund-Levander, Forsberg and Wahren17]. Blood samples were drawn by a study nurse and were evaluated for HIV infection using immunochromatographic rapid tests according to the national guidelines [18]. Malaria infection was confirmed using thick blood smears and/or rapid diagnostic antigen tests (SD BIOLINE Malaria Ag P.f/Pan). A full hemogram was performed at MNRH haematology laboratory using an automated haemonalyser (Sysmex® Automated haematology analyser XN series – XN 1000) following standard procedures. Anaemia was defined as a haemoglobin level of <13.0 grams per decilitre (g/dl) for males and <12.0 g/dl for females and graded as mild (11.0–12.9 g/dl for men and 11.0–11.9 g/dl for females), moderate (8.0–10.9 g/dl for both sexes) and severe (<8.0 g/dl for both sexes) [19]. Anaemia was classified as hypochromic if the mean corpuscular haemoglobin (MCH) was <24 picograms (pg) [Reference Frewin, Henson and Provan20]. Microcytosis was defined as a mean corpuscular volume (MCV) of <76 femtolitres while macrocytosis was an MCV of >96 fl [Reference Frewin, Henson and Provan20]. The MCH of <24 pg and MCH of <76 are considered appropriate cut-offs in screening for iron deficiency anaemia [Reference Frewin, Henson and Provan20]. The CD4 and CD8 T-cell counts and the CD4:CD8 ratio were measured by flow cytometry (BD FACSCalibur™). Sputum bacillary load was graded as very low (Xpert MTB/RIF cycle threshold (Ct) >28 or 1–9 acid-fast bacilli (AFBs)/100 fields on smear microscopy), low (Ct 22–28 or 1–9 AFBs/10 fields), medium (Ct 16–22 or 1–10 AFBs/field) and very high (Ct <16 or >10 AFBs/field) [Reference Lawn and Nicol21, Reference Enarson22].
Statistical analysis
Data were analysed using Stata 14 (StataCorp, College Station, TX, USA). Categorical variables were compared among people with and without anaemia using Pearson's χ 2 or Fischer's exact tests as appropriate. Continuous variables were compared using the Mood's median test. Variables with P < 0.2 in bivariate analysis were entered into multivariate logistic regression model. We performed multivariable logistic regression analysis to determine factors that are independently associated with anaemia. Antiretroviral therapy (ART) use, and duration of weight loss and fever were dropped during model building to achieve a parsimonious model. Statistical significance was set, a priori, at P < 0.05 at the 95% confidence interval.
Ethical approval and consent to participate
In the primary study, participants provided written informed consent before study procedures. The study was approved by the School of Medicine Research and Ethics Committee of Makerere University College of Health Sciences (REC REF 2017-087). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
Of the 363 patients in the primary database, 358 (98.6%) had a haemoglobin measurement and were included in this analysis.
Characteristics of TB patients with and without anaemia
Of 358 people with TB, 220 (61.5%) were males, 140 (39.1%) were aged 25–34 years, and 127 (35.5%) were HIV co-infected. Anaemia was prevalent among 210 (58.7%, 95% CI 53.4–63.8). As shown in Table 1, a higher proportion of patients with anaemia were cigarette smokers (21.6% vs. 33.8%, P = 0.012), HIV-positive (24.0% vs. 43.3%, P < 0.001) and reported night sweats (63.5% vs. 73.8%, P = 0.037), had hyperthermia (8.2% vs. 16.7%, P = 0.010) and a longer duration of fever (median days (IQR)) (14 (7–30) vs. 21 (14–52.2), P = 0.040). They also had very high sputum bacillary load (22.5% vs. 33.3%, P = 0.020) but lower median (IQR) BMI (19.5 (17.7–21.4) vs. 17.6 (16.0–19.7) kg/m2, P < 0.001), CD4 (564 (380–794) vs. 344 (146–613) cell/mm3, P < 0.001) and CD8 (460.5 (308.5–679.5) vs. 385 (225–585) cell/mm3, P = 0.040) T-cell counts, MCV (80.7 (73.8–88.9) vs. 76.3 (70.0–86.6) fl, P = 0.010), and MCH (27.4 (24.9–29.4) vs. 24.9 (22.6–27.5) pg, P < 0.001). However, more people without anaemia reported weight loss (32.4% vs. 18.1%, P = 0.002).
Table 1. Characteristics of TB patients with and without anaemia in Uganda

a Cut-offs are for normal adult Ugandans [Reference Nanzigu47].
Severity of anaemia among people with TB in Uganda
Of the 210 people with anaemia, 85 (40.5%, 95% CI 33.8–47.4) had mild, 101 (48.1%, 95% CI 41.2–55.1) had moderate and 24 (11.4%, 95% CI 7.5–16.5) had severe anaemia. As shown in Table 2, most people with severe anaemia were HIV-positive (75.0%) compared to those with moderate (48.5%) and mild (28.2%) anaemia (P < 0.001). Similarly, most people with severe anaemia had low CD4 T-cell counts (83.3%) and CD4:CD8 ratio (79.2%) than those with moderate (67.3% and 43.6%) and mild anaemia (40.0% and 22.4%), respectively, P < 0.001.
Table 2. Characteristics of TB patients with mild, moderate and severe anaemia
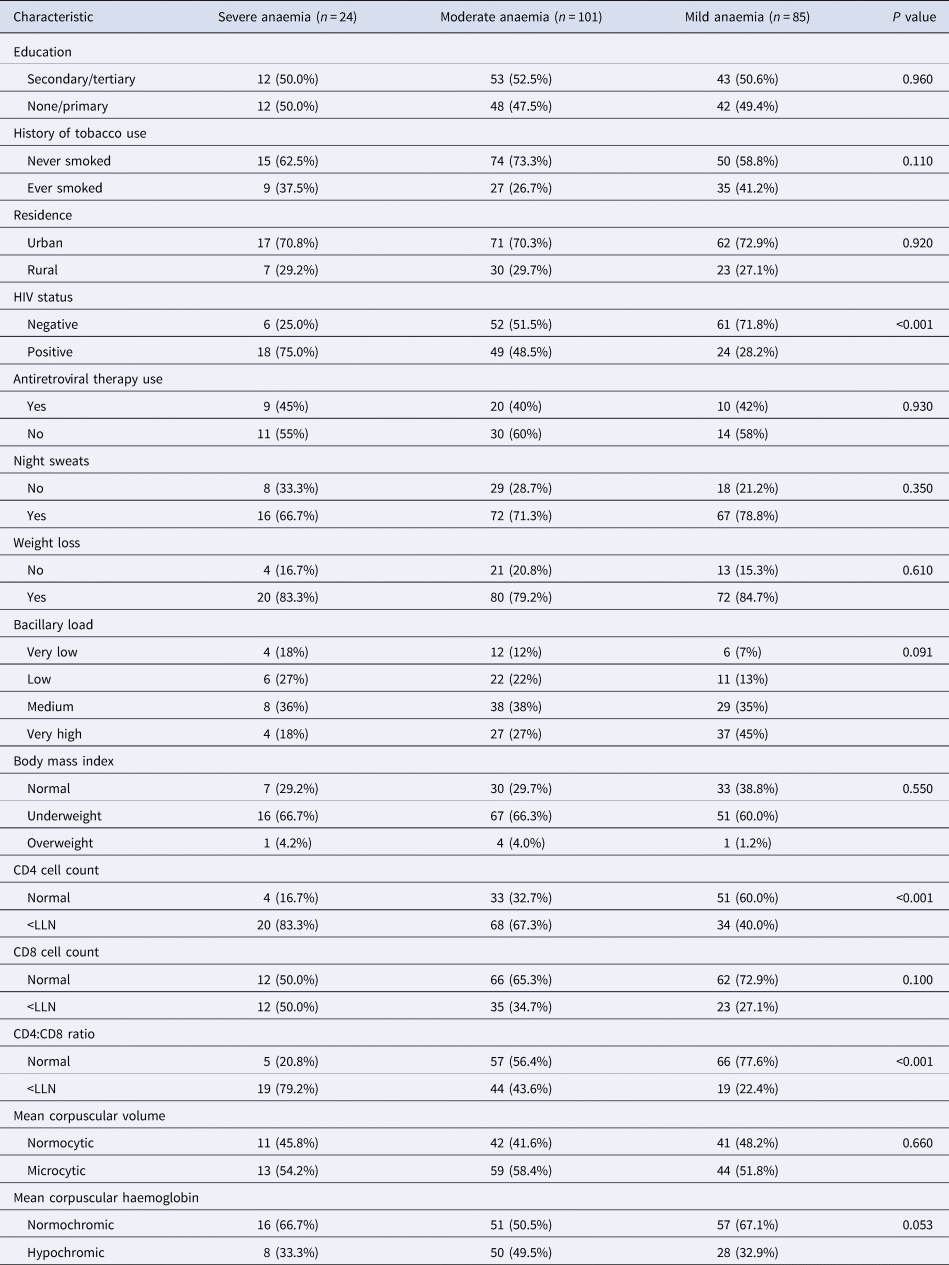
LLN, lower limit of normal for Ugandans (normal ranges for CD4 and CD8 counts and CD4:CD8 ratio were 418–2105 cells per microliter (μl), 256–1619 cells/μl, and 0.52–4.1, respectively [Reference Nanzigu47]).
Factors independently associated with anaemia among people with TB in Uganda
As shown in Table 3, at multivariate analysis, anaemia was associated with being underweight (odds ratio (OR) 2.93, 95% CI 1.70–5.05, P < 0.01), low CD4:CD8 ratio (OR 2.54, 95% CI 1.07–6.04, P = 0.035) and hypochromia (OR 4.23, 95% CI 2.17–8.25, P < 0.01).
Table 3. Multivariate logistic regression model for factors associated with anaemia among people with TB in Uganda

LLN, lower limit of normal for Ugandans (normal ranges for CD4 and CD8 counts and CD4:CD8 ratio were 418–2105 cells per microliter (μl), 256–1619 cells/μl and 0.52–4.1, respectively [Reference Nanzigu47]).
Discussion
In this study, we evaluated the prevalence and factors associated with anaemia among people with TB in Uganda. Almost 60% of people with TB had anaemia, 60% of whom had moderate or severe anaemia. This result is in agreement with the global estimate of the prevalence of anaemia in TB of 62% [Reference Barzegari5]. The high proportion of TB patients with anaemia should be of public interest. Anaemia adversely affects TB outcomes in several ways. First, data from the general population suggest that anaemia is associated with all-cause mortality independent of age, sex and cardiovascular disease [Reference Liu23]. It is therefore not surprising that anaemia (with or without iron deficiency) has been associated with a threefold risk of mortality among people with TB [Reference Isanaka9]. Second, anaemia is emerging as an important risk factor for TB infection and disease in HIV-negative and HIV-positive individuals in a dose-dependent manner, regardless of the type of anaemia [Reference Gelaw, Getaneh and Melku4, Reference Abioye24, Reference Kerkhoff25]. Lastly, anaemia in TB and the associated systemic inflammation do not invariably resolve on TB therapy, and could pose risk for other complications even after TB cure [Reference Minchella6, Reference Gil-Santana26, Reference Kassa27]. Therefore, there is the need for intensification of population-wide interventions to reduce the burden of anaemia in TB and HIV high-burdened countries as part of the strategies to reduce the incidence of TB. Additionally, guidelines for the management of anaemia in TB are needed to improve TB outcomes in patients with anaemia. Also, prospective studies are desirable to further characterise the evolution of anaemia and its complications (if any) after TB cure.
In the present study, anaemia was associated with night sweats, a longer duration of fever, low BMI, hyperthermia, high sputum bacillary loads, HIV co-infection, and low CD4 and CD8 counts at bivariate analysis. These findings suggest that anaemia is associated with the features of severe TB disease. Low BMI, elevated temperature and night sweats are part of a well-validated TB severity score (the Bandim TBscore) [Reference Wejse28]. Moreover, HIV co-infection with severe immune suppression and high baseline mycobacterial loads are established risk factors for mortality in drug-susceptible and drug-resistant TB [Reference Alemu29, Reference Teferi30]. Subsequent studies should evaluate the utility of integrating haemoglobin levels in the existent TB severity scores since clinical evaluation of anaemia (pallor) does not correlate well with haemoglobin measurements [Reference Rudolf31, Reference Pefura-Yone32]. Similar to our findings, de Mendonça et al. [Reference de Mendonça8] recently reported anaemia to be associated with severe TB, low BMI and HIV co-infection in Brazil. We did not find any association of anaemia with advanced age and female sex as was reported by Lee et al. [Reference Lee33] in South Korea. This could be because they had a relatively older population with a median age of 44 years (compared to the >60% of our population which was aged ≤35 years). It is unclear why more people without anaemia reported weight loss than people with anaemia in our study. However, this was through self-reports, and we did not objectively confirm the weight loss. Self-reports are therefore not very reliable.
The association of TB-related anaemia with low BMI, as observed in our multivariate analysis is consistently observed in literature [Reference Oliveira34, Reference Mukherjee35]. Malnutrition is likely to be the common cause for both anaemia and low BMI in TB [Reference Feleke, Feleke and Biadglegne36]. It is also hypothesised that abnormalities in appetite mediators – leptin and ghrelin – and inflammatory cytokines in TB could concurrently cause low nutrient intake, iron trapping in the reticuloendothelial cells and alter fat metabolism [Reference Zheng37, Reference Hella38]. The association of anaemia with a low CD4:CD8 ratio, independent of HIV infection, is interesting. A low CD4:CD8 ratio has been associated with altered immune responses, immune senescence and chronic inflammation and may predict mortality and morbidity in individuals with and without HIV [Reference McBride and Striker39]. The role of the CD4:CD8 ratio is not well established in TB. One meta-analysis has shown that newly diagnosed TB patients have reduced CD4:CD8 ratios compared to normal controls [Reference Yin40]. The CD4:CD8 ratio may also predict TB drug resistance [Reference Baluku41, Reference Li42]. The relationship between the CD4:CD8 ratio and anaemia has been evaluated mostly in the context of iron deficiency. Iron deficiency anaemia is associated with a low CD4:CD8 ratio which improves on iron supplementation in children [Reference Mullick43–Reference Das45]. Iron is essential for proliferation and activation of CD4+ helper T-lymphocytes and intracellular iron deficiency could impair the function of the enzymes that drive the metabolic and redox reactions involved in these processes [Reference Cronin46]. Therefore, our finding suggests iron deficiency as a possible cause of low CD4 counts (hence low CD4:CD8 ratio) and this seems to occur in a dose-dependent manner since more patients with severe anaemia had low CD4 T-cell counts and CD4:CD8 ratios than those with a mild form. This relationship between anaemia severity and T-cell counts was not observed with CD8 cell counts.
From our study, it is difficult to delineate the cause of anaemia. Microcytosis and hypochromia were observed to be a commoner among patients with anaemia as has been shown elsewhere [Reference de Mendonça8, Reference Lee33, Reference Mukherjee35]. This could suggest anaemia of chronic disease or iron deficiency which account for 50% and 20% of anaemia in TB, respectively [Reference Barzegari5]. However, nutritional causes, such as iron deficiency, are likely in our study population as evidenced by the association of anaemia with low BMI and low CD4:CD8 ratio as discussed above. The cross-sectional nature of our study is unable to establish the direction of the relationship between anaemia and TB; that is, whether anaemia was a risk factor for TB or TB was the cause of anaemia. Considering the relatively short duration of most symptoms (≤30 days), it is reasonable to suggest that anaemia preceded clinical TB disease. However, this is best ascertained by prospective studies.
Our study has limitations. First, we could not evaluate the association between anaemia and radiological manifestations because the data were not available. This would otherwise better characterise TB severity. Second, confounders such as helminth co-infection, gastrointestinal bleeding and dietary habits were not evaluated for. Nevertheless, we were able to assess for association with HIV, haemoparasites (malaria) and drugs (ART and cotrimoxazole). Another limitation is that the study was conducted at a referral facility and the results could be affected by referral bias, in which case patients with severe TB and/or anaemia could have been preferentially referred for care at the NTTC. This limits the generalisability of our study. However, at the NTTC, <30% of patients are referral cases, and a minority of patients (28%) were from rural areas (presumed to have been referred). Additionally, the hospitalisation status and history of previous transfusions among the participants were not documented, which could be confounders. Moreover, some variables such as the clinical symptoms and symptom duration were self-reported and could be affected by recall bias. Lastly, data on iron studies, serum hepicidin and cytokines were not available to enable us to type the anaemia.
Conclusion
The prevalence of anaemia was high among people with bacteriologically confirmed TB at the NTTC in Uganda. Majority of the patients have moderate to severe anaemia. Anaemia was associated with the features suggestive of severe TB disease: high bacillary load, night sweats, low BMI, longer duration of fever, hyperthermia, HIV co-infection, low T-cell counts and low CD4:CD8 ratio. There is the need to include anaemia in TB severity scores. Guidelines for managing anaemia in TB are needed in low-income settings where facilities for establishing specific aetiology of the anaemia are not readily available.
Acknowledgements
None.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Data availability statement
Datasets used in this analysis are available from the corresponding author upon reasonable request.








