INTRODUCTION
In order to take appropriate measures in diagnostics, prophylaxis and therapy of human papillomavirus (HPV) infection in a specific geographical area, it is necessary to determine the epidemiological pattern of HPV genotypes distributed at the local level. Bosnia and Herzegovina (B&H) is one of nine Central and Eastern European (CEE) countries (Albania, Bulgaria, Croatia, FYR of Macedonia, Montenegro, Romania, Serbia, Slovakia) with an established and opportunistic cytology-based cervical cancer screening programme with complementary high-risk (HR) HPV DNA testing [Reference Poljak1, Reference Rogovskaya2]. According to the latest recommendations [Reference Poljak3], this model of cervical cancer prevention should be progressively modified to an organized screening programme with the immediate implementation of universal HPV vaccination into the national immunization schedule. Nevertheless, HPV vaccination has already been integrated into the national immunization programme in three of the CEE countries: Bulgaria (from 2012), FYR Macedonia (from 2009) and Romania (from 2008), primarily targeting females aged 12 years [Reference Poljak3]. In B&H, both prophylactic vaccines have been registered: Cervarix® (GlaxoSmithKline, UK, since 2007) and Gardasil® (Merck & Co., USA, since 2008), and are commercially available currently for voluntary immunization. As those vaccines protect against the two main HR HPV genotypes (HPV-16, HPV-18), it is necessary to assess whether these two HR HPVs are dominant in the study population, or if vaccines directed against a broader spectrum of HR HPVs could be more effective in this region. A nine-valent next-generation HPV vaccine, code-named V503 (Merck & Co., USA), is at the final stage of clinical trials (Phase III), designed to protect against an additional five HR HPVs (HPV-31, -33, -35, -45, -52, -58), as an upgrade of Gardasil vaccine (Merck & Co.) [4]. In order to predict the potential coverage of vaccination HR HPV types and to better define choice of secondary prevention of cervical cancer in B&H, studies based on the distribution of individual HPV types targeted by vaccines are crucial. The aim of this study was to determine the distribution of HPV genotypes in a group of Bosnian-Herzegovinian women with proven HPV infection and abnormal cervical cytology, as well as to assess the potential coverage of identified HPV genotypes by vaccines.
METHODS
This section presents a brief overview of the design and methods used in the study. More detailed data have been described elsewhere [Reference Salimović-Bešić5].
Study subjects
A group of women (n = 105) with positive cervical cytology ranging from atypical squamous cells of undetermined significance (37/105), low-grade squamous intraepithelial lesion (33/105) to high-grade squamous intraepithelial lesion (35/105), and HR HPV infection detected by HPV DNA screening test were included in the study. The average age of the women was 36·6 ± 9·5 years (range 19–62 years). Women were mainly recruited from outpatient clinics (the Public Institution Centre for Women and Maternity Health Care of Sarajevo Canton, B&H) and some gynaecologists in private practice in Sarajevo (B&H), who were referred to University Clinical Centre – Sarajevo (B&H) or the Institute for Biomedical Diagnostics and Research ‘Nalaz’ in Sarajevo (B&H) for HPV testing.
Conventional Pap smears were taken by gynaecologists and examined by experienced cytologists independently of HPV testing.
Specimen collection for laboratory analysis
Cervical specimens were collected from June 2010 to December 2012 with digene Cervical Sampler-STM (Qiagen, USA) (88/105), Abbott Cervi-Collect Specimen Collection kit (Abbott Molecular, Germany) (14/105) or ThinPrep Pap Test PreservCyt Solution (Cytic Corporation, USA) (3/105). All samples were stored for up to 7 days at +4 °C, or for longer at −20 °C/−70 °C until testing. HPV testing was performed at: (a) University Clinical Centre – Sarajevo, B&H, Department of Clinical Microbiology – Division of Virology, (b) Institute for Biomedical Diagnostics and Research ‘Nalaz’ in Sarajevo, B&H.
DNA-based detection assays
Screening for HR HPV infection was performed by one of the two clinical assays: Hybrid Capture 2 assay (HC2; Qiagen Corporation, USA ) done with a HR HPV probe cocktail and the Abbott RealTime High Risk HPV test (Abbott Molecular), in accordance withthe manufacturers' protocol.
DNA-based genotyping assays
Clinical material included in the study was separated into appropriate aliquots required for extraction of total nucleic acids. Specimens were stored at a temperature of +4 °C for up to 7 days, or for longer at −20 °C/−70 °C until genotyping assay was performed.
Total nucleic acid extraction was performed by using NucliSENS® miniMAG™ Magnetic Extraction kit (bioMérieux, France) from sample aliquots separated before the HPV DNA screening test was performed. The 400 μl aliquots of samples collected with the digene Cervical Sampler-STM (Qiagen) and the 1 ml aliquots of samples collected with the Abbott Cervi-Collect Specimen Collection kit (Abbott Molecular) were transferred for extraction. Samples taken in ThinPrep Pap Test PreservCyt Solution (Cytic Corporation) were separated in 5 ml aliquots, centrifuged for 12 min at 1125 g, then 4 ml of supernatant were discarded and the pellet was resuspended in the remainder of the 1 ml supernatant. DNA/RNA was eluted in 55 μl elution buffer and stored for a week at +4 °C or a month at −20 °C prior to testing.
HPV genotyping was performed by the following assays according manufacturer's instructions: HPV High Risk Typing Real-TM test (Sacace Biotechnologies, Italy) was used for qualitative detection and genotyping of 12 HR HPVs: HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59 in the cervical swabs. The test is based on multiplex real-time PCR amplification run in four tubes for each sample. Each tube contained primers directed against regions of three HPV types with the human b-globin gene used as internal control. The 20 μl of total nucleic acid extracts per sample were used in four PCR reactions (8 μl master mix and 5 μl eluate made 13 μl of each of four PCR mixes).
Genotyping of five samples with indeterminate results obtained by High Risk Typing Real-TM (Sacace Biotechnologies) was performed by a reverse line probe hybridization diagnostic kit INNO-LIPA HPV Genotyping Extra (Innogenetics, Belgium). The 10 μl of total nucleic acid extracts were used for the analysis. The assay works with a short PCR fragment (SPF10-PCR) designed to discriminate a broad spectrum of HPV types by reverse line probe hybridization, thus allowing the detection of 54 HPV types and the identification of 28 of them.
Statistical analysis
DStatistical analysis was performed with SPSS for Windows v. 15·0 (SPSS Inc., USA). escriptive statistics were expressed by frequency, arithmetic mean, standard deviation (s.d.), minimum and maximum values and percentages. The relationship of cytological diagnosis and HPV type was evaluated by χ 2 test of independence of variables. Significance was based on P < 0·05.
Type-specific calculations were made without taking into account multiple HPV infections, but considering each identified HPV infection as a single infection.
Ethical standards
This study was conducted according to the principles expressed in the Declaration of Helsinki, and was approved by the ethical review boards of the University Clinical Centre – Sarajevo and the Institute for Biomedical Diagnostics and Research ‘Nalaz’ in Sarajevo (B&H). In order to protect the identity of the patients, all clinical samples used in the study were coded and tested anonymously.
RESULTS
Analysis of HPV genotypes
In the studied population of women (n = 105), 16 different HPV types were detected. Fifteen HPV types were identified by the methods used, while one remained unidentified (HPV-X) (Table 1). On the basis of viral oncogenic potential, 12/15 identified HPV types belonged to the HR HPV genotypes group, 2/15 were probable HR genotypes, and 1/15 genotype was placed into the low-risk HPV genotype group (Table 1). The frequency (f) of individual HPV genotypes was calculated so that each detected genotype was considered as a separate infection. In fact, this part of the data analysis did not take into account the presence of multiple HPV infections. The prevalence of different HPV genotypes was calculated according to the cumulative number of infections caused by each individual HPV type detected in the study. The most common genotype was HPV-16 detected in 32·6% (48/147), followed by HPV-31 in 14·3% (21/147), and HPV-51 in 9·5% (14/147) of women. HPV-18 was the fourth most common genotype in 7·5% (11/147) of women (Table 1). The overall frequency of infections caused by HR HPV-16 and/or HPV-18, covered by vaccines (Gardasil and Cervarix) was 40·1% (59/174) and significantly lower (χ 2 = 5·7, P = 0·017) than the overall frequency of all other HPVs detected in the study. By contrast, the frequency of seven HR HPV genotypes (HPV-16, -18, -31, -33, -45, -52, -58) targeted by the vaccine V503, with the exception of two low-risk (HPV-6 and HPV-11) types (Merck & Co.) was 68·0% (100/147) and significantly higher (χ 2 = 19·109, P < 0·001) than the total frequency of the other HPVs detected. In one case (0·7%, 1/147), HPV genotype remained undetermined (HPV-X), although the presence of HPV was confirmed by the two methods [INNO-LIPA HPV Genotyping Extra (Innogenetics), and HC2 using an HR HPV probe cocktail (Qiagen)].
Table 1. Distribution of human papillomavirus (HPV) types in women grouped according to their cervical cytological diagnosis

HPV-53, HPV-66 and HPV-70 were identified by INNO-LiPA HPV Genotyping Extra (Innogenetics, Belgium). In the case of HPV-X, although the high-risk HC2 (Qiagen, USA) screening test showed a positive result the HPV High Risk Typing Real-TM (Sacace Biotechnologies, Italy) , genotyping assay failed to identify this HPV type. Likewise, within the INNO-LiPA HPV Genotyping Extra (Innogenetics) assay, specific SPF10-PCR HPV product was obtained, but HPV type was not ultimately identified.
Multiple HPV infections
Of the tested women, multiple infections (consisting of 2–5 different HPVs) were present in 26·7% (28/105). The most prevalent of these were double infections (18·1%, 19/105). Frequency of multiple infections decreased with increasing numbers of HPVs simultaneously present in infection (Table 2).
Table 2. Frequency and percentage of multiple human papillomavirus (HPV) infections
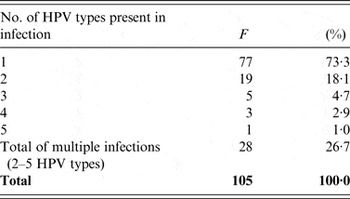
F, Frequency of single and multiple HPV infections and their prevalence (%) in 105 tested women.
The composition of multiple infections, as well as the HPV DNA test used for viral identification, and cervical cytology diagnosis for each case are listed in Table 3.
Table 3. Multiple human papillomavirus (HPV) infections and HPV DNA tests used for their detection in women listed by increasing severity of cervical cytology
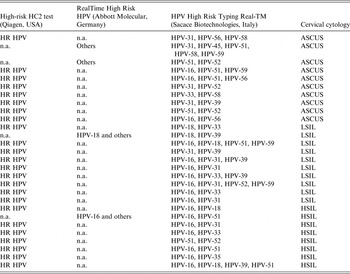
HR, High risk; n.a., not applied; others, any HPV type in the group of the ten HR HPV types (HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59) and two probable HR HPV types (HPV-66 and HPV-68) detectable by RealTime High Risk HPV test (Abbott Molecular), ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSI, high-grade squamous intraepithelial lesion.
Analysis of HPV genotypes according to cervical cytology
In cytological groups of women with atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL), respectively, 12 different HPV genotypes (f ASCUS = 53 and f LSIL = 49) were found. Of these, one undetermined genotype (HPV-X) was present in a woman with a low-grade cervical lesion. Eleven different HPVs (f HSIL = 45) were identified in the group of women with high-grade squamous intraepithelial lesion (HSIL). The most common genotype in all three groups of women separated according to cervical cytology was HPV-16, detected in the ASCUS group in 20·8% (11/53), in the LSIL group in 30·6% (15/49) and in the HSIL group in 48·9% (22/45). In the group of women with ASCUS cytology index, HPV-16 was followed by HPV-31 (13·2%, 7/53) and HPV-56 (11·3%, 6/53), while genotype HPV-18 equal to HPV-58 was the sixth the most common HPV genotype in the group (5·7%, 3/53). After HPV-16, in the group of women with LSIL cytology index, HPV-31 was present in 14·3% (7/49), and then each of HPV-18 and HPV-39 genotypes were identified in 10·2% (5/49). In women with high-grade cervical lesions, as in the previous two groups of women, HPV-31 was the second most prevalent genotype (15·6%, 7/45), followed by HPV-51 in 11·1% (5/45) and HPV-18 in 6·7% (3/45) (Table 1).
The dependence of cytological diagnosis (ASCUS, LSIL or HSIL) and infections caused by HPV-16 and -18, or HPV-16, -18, and -31, respectively, compared to other HPVs was observed (χ 2 = 9·7, P = 0·008 and χ 2 = 9·59, P = 0·008). Global percentage of infections caused by HPV-16 and -18, or HPV-16, -18 and -31, respectively, increased with severity of cervical cytology, while global proportion of infections caused by other HPVs declined (Fig. 1).
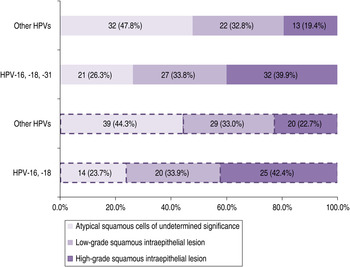
Fig. 1. Infections caused by HPV-16, -18 and HPV-16, -18, -31, respectively, vs. other HPV types in women with atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion or high-grade squamous intraepithelial lesion of the cervix.
However, development of cervical cytological abnormalities was independent of the presence of multiple infections (χ 2 = 0·598, P = 0·741) (Fig. 2).

Fig. 2. Prevalence of single and multiple infections in women with atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion or high-grade squamous intraepithelial lesion of the cervix. Independence of cervical cytological diagnosis and infections caused either by a single HPV type or 2–5 HPV types simultaneously present. Numbers in the chart represent frequencies and percentages of given HPV infection according to cytology.
DISCUSSION
Determination of circulating HPV types in a population can provide an early measure of vaccine impact. In particular, it is important in countries where organized screening programmes for the prevention of cervical cancer have not yet been established.
Despite the relatively small number of subjects involved in the study, reliable statistical analysis of the data was made. Thus, the most common genotype in the study was HPV-16, followed by HPV-31 and -51, similar to the distribution pattern published from Turkey [Reference Yuce6]. Infections caused by HPV-18 and -39 occupied fourth place.
According to these results and data from previous studies of B&H, regional studies and meta-analysis [Reference Salimović-Bešić5, Reference Vranic, Gravitt and Hardick7–Reference de Sanjose12], HR HPV-31, as well as HPV-16, plays a key role in the prevalence of HPV infection. The new generation of HPV vaccines directed against this genotype may have a greater public health contribution to protection of the development of cervical abnormalities than ones without it. Currently available vaccines targeted against two high-risk genotypes (HPV-16 and -18) would cover 40·1% of cases of HPV infection in women involved in this study. The combination of HR HPV-16, -18, -31, -33, -45, -52 and -58, contained in the nine-valent vaccine designated V503, would cover 68·0% of infections, which is significantly higher coverage compared to the prevalence of other HPVs. The latest study on the potential impact of V503 vaccine [Reference Serrano13], has shown that almost 90·0% of cases of invasive cervical cancer worldwide could be prevented. If this vaccine reaches the same level of efficiency as the previous two vaccines (Gardasil and Cervarix), the global incidence of cancer would be significantly reduced. In February 2014, the Food and Drug Administration (FDA) accepted Merck's application for V503, thus setting the stage for a near-term approval.
HPV-16 and HPV-31 were also the most common genotypes in all three categories of cervical cytological changes and individually have shown rising percentage with the severity of cytological changes (from ASCUS to HSIL). The prevalence of HR HPV-18, -33 and -39 increased from ASCUS to LSIL, and then declined from LSIL to HSIL. HR genotypes HPV-45, -52, -56 and -59 decreased from ASCUS to HSIL gradually. HPV-18 was not among the dominant genotypes, but in a previous study from B&H, this genotype showed the highest oncogenic activity (100%) in the same population of women [Reference Salimović-Bešić5].
With the growing cervical cytology index of LSIL to HSIL, as seen in a study from Croatia [Reference Grahovac9], an increasing prevalence of genotypes HPV-16, -18, -31 and -33 was recorded, while the percentage of genotypes HPV-45, -52 and -58 decreased.
Recently, a meta-analysis of cross-sectional HR HPV-type distribution in 115 789 HPV-positive women [Reference Guan10] with normal cervical cytology or ASCUS, LSIL and HSIL cytological changes, as well as groups of women with histologically confirmed diagnoses of CIN1, CIN2, CIN3 and invasive cervical cancer was conducted. Data were obtained from 423 PCR-based studies worldwide. Large differences in the distribution of HPV genotypes in women with normal cervical cytology, ASCUS, LSIL or CIN1 were not observed. However, HPV-16 positivity increased gradually, in the following order: normal cytology/ASCUS/LSIL/CIN1 (20·0–28·0%), over CIN2/HSIL (40·0/47·0%) to CIN3/invasive carcinoma of the cervix (58·0/63·0%). We concluded in view of this and results of the previous meta-analysis [Reference de Sanjose12], that the HPV-16 genotype, in particular, as well as HPV-18 and HPV-45, deserve special attention in the screening of HPV infection.
Presence and consequences of multiple HPV infections are still unclear. Although the accumulation of HPVs is often observed [Reference Salimović-Bešić, Poljak and Kocjan14–Reference Mendez17], follow-up studies of infected women suggest that the presence of multiple HPV genotypes has no effect on the course of infection [Reference Plummer18].
There werre 26·7% of multiple infections detected in the study, with dual infections prevailing. However, the development of cytological abnormalities in the categories of ASCUS, LSIL and HSIL was not statistically dependent on such infections.
The emergence of mixed infections is generally explained by way of transmission of HPV types and association with risk factors [Reference Thomas16, Reference Mendez17], such as the number of lifetime sexual partners [Reference Salimović-Bešić19], sexual behaviour of partners [Reference Vaccarella20, Reference Winer21], and recent sexual partners [Reference Winer22], or that it arises as a result of immunological tolerance [Reference Kleter23].
Range of genotypes identified in the study depends on the capability of the test used for viral typing. In the study, the main typing test (HPV High Risk Typing Real-TM, Sacace Biotechnologies) was chosen to identify the majority of HPV genotypes detected by HPV DNA screening tests [92·3% (12/13) for the HC2 test (Qiagen) and 85·7% (12/14) for RealTime High Risk HPV (Abbott Molecular)]. By this method, the number of unspecified HPV genotypes was relatively low (3·4%, 5/147).
A single HPV-X suggests that typing tests used in the study were capable of identifying at least one viral genotype in 99·3% (146/147) of cases.
As in other studies [Reference Lie24–Reference Poljak26], false positivity of the presence of HR HPV infection was obtained at a screening test (HC2; Qiagen). Cross-reactivity with genotypes HPV-53 (probably a HR genotype) and HPV-70 (low-risk genotype in two cases) was found due to hybridization with complete viral genomic probes supplied in the kit.
In conclusion, vaccines targeted against HR HPV-16, -18 and -31 will be of great importance for the prevention of cervical disease in B&H. Due to the limited number of such studies originating from this area, it is necessary to monitor the circulation of the carcinogenic viruses on a regular basis with a large number of patients included.
ACKNOWLEDGEMENTS
This work was supported by the Federal Ministry of Education and Science of Bosnia and Herzegovina (05-39-4069-1/11).
DECLARATION OF INTEREST
None.










