INTRODUCTION
Despite the advent of newer antimicrobials, meningococcal infections remain a leading cause of morbidity and mortality. The infection has also gained importance because of the link with the Hajj, the Muslim holy pilgrimage. Serogroup W135 has recently been associated with an international outbreak that was directly linked with the Hajj. Serogroup Y tends to affect older adults and is responsible for one third of meningococcal infections in the United States. The number of cases involving serogroup Y has increased over the years and is linked with pneumonia. Meningococci can spread rapidly from person to person, especially in closely confined populations such as schools or military barracks.
Neisseria meningitidis has been implicated in outbreaks and epidemics worldwide [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Raja2]. It is endemic in temperate regions with seasonal increases in winter and spring. A different pattern of endemicity with periodic outbreaks covering large geographical areas has been observed in countries of sub-Saharan Africa. In India outbreaks have been recorded from as far back as 1883; most of the episodes have been caused by serogroup A, and some by serogroup C. In Delhi, the earliest recorded outbreak was in 1935. More recently these have occurred with a regular frequency of about once every two decades: in 1966–1967, 1985–1986 and most recently in 2005–2006 [3]. This could possibly be explained by travel, migration, displacement of population and decreasing herd immunity. Herd immunity wanes with time and is suggested as a possible cause for recurrence of epidemics of N. meningitidis [Reference Taha4].
In the spring/summer of 2005 the first of several cases of meningitis was reported from Old Delhi. The Government declared an outbreak, which persisted over two periods, from April–July 2005 (4 months) and January–March 2006 (3 months). A total of 444 meningococcal cases with 62 (13·9%) deaths occurred during the outbreak in April–July 2005 in and around Delhi. The WHO had reported an overall case fatality of 11·9% as of June 2005 (http://www.who.int/csr/don/2005_06_14/en/index.html). Subsequently the outbreak spread to the entire National Capital Region, which includes New Delhi and the territories of states which adjoin the capital city borders. In January–March 2006 there were 177 cases with 17 (9·6%) deaths. In total, 128 cases had microbiological evidence of infection and 40 were culture positive from the city [Reference Manchanda, Gupta and Bhalla5]. All age groups were affected, the commonest being 14–45 years. There was a male preponderance of 87·3%. Maximum mortality (62%) was in the 15–30 years age group [Reference Raja2, Reference Singhal6]. Over the years the mortality associated with meningococcal disease as reported by the Central Bureau of Health Investigation, India, has been 50/397 (12·6%) in 2002, 48/325 (14·8%) in 2003, 20/249 (8%) in 2004 (B. K. Das et al., unpublished data).
During this outbreak the WHO criteria for diagnosis of meningococcal disease, as recommended by our National Institute of Communicable Diseases (NICD), was used. The criteria for a ‘probable’ case is one that meets clinical suspicion of meningococcal disease in conjunction with turbid CSF (with or without positive Gram stain) or with an ongoing epidemic and epidemiological link to a confirmed case. A ‘confirmed’ case was a clinically suspected or probable case with laboratory confirmation by CSF antigen detection or culture. This case definition differs from that given by the United States Centers for Disease Control (CDC) [7]. On the basis of the WHO criteria, 55 patients were diagnosed as confirmed/probable cases from our hospital.
Reports showing the current epidemiology and antimicrobial susceptibility pattern of N. meningitidis isolates from India are scarce. The objective of this report was to describe the outbreak of meningococcal disease in relation to the laboratory diagnostic methods, antimicrobial susceptibility and demographic data based on admissions to Safdarjung Hospital, New Delhi. This is a 1570-bed, tertiary-level government hospital that serves the National Capital Region (region of New Delhi and its adjacent territories).
METHODS
Samples of blood (22), petechial smears (23) and CSF (41) for culture, Gram stain and latex agglutination test (LAT) were received. A portion of the CSF sample taken at emergency admission was immediately inoculated onto chocolate agar slants. The CSF samples collected in the wards were inoculated onto chocolate agar and sheep blood agar (BD Difco, Franklin Lakes, NJ, USA). All media were incubated at 37°C with 5–10% CO2, for up to 72 h. They were examined daily for evidence of growth. The characteristic small, moist, transparent colonies with glistening surfaces were examined by Gram stain, oxidase test and were confirmed by grouping antisera, N. meningitidis antisera (set A–D) (Difco, Difco Laboratories, Detroit, MI, USA) [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1].
Gram-stained smears from a centrifuged deposit of the CSF were observed. The supernatant was subjected to LAT using Pastorex meningitis latex agglutination kit (Bio-Rad, Hercules, CA, USA), which detects the soluble antigen of N. meningitidis A, B/E. coli K1, C, Y/W135, Haemophilus influenzae type b, Pneumococcus and Streptococcus group B. Slit skin smears obtained were fixed in alcohol and Gram-stained. The presence of extracellular and intracellular Gram-negative diplococci was considered significant. Blood cultures were processed by standard method [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1]. The bottles were incubated at 37°C with 5–10% CO2 for 24 h and subcultured onto sheep blood agar and chocolate agar with brain heart infusion agar base (Difco).
The antimicrobial susceptibility testing was performed and interpreted as previously described [Reference Vazquez8]. E strips were placed on the plates and incubated at 35°C in 5% CO2 (except the plate with the macrolide, azithromycin E strip). Agar dilution test was performed to confirm the results of E strip, as per Clinical and Laboratory Standards Institute (CLSI 2007) criteria [9]. Antimicrobials tested were azithromycin (0·007–64 μg/ml), cefotaxime (0·0003–0·03 μg/ml), ciprofloxacin (0·0007–16 μg/ml), levofloxacin (0·0007–16 μg/ml), ofloxacin (0·0007–16 μg/ml), penicillin G (0·007–2 μg/ml) and rifampicin (0·007–64 μg/ml). The quality control was performed using S. pneumoniae ATCC 49619 and E. coli ATCC 25922 [Reference Vazquez8, 9].
The CrgA gene (Fig. 1) is involved in the regulation of adhesion of N. meningitidis to target cells. Polymerase chain reaction (PCR) was performed (My cycler, Bio-Rad) targeting the gene for this region as per standard protocol [Reference Taha10–Reference Borrow12].
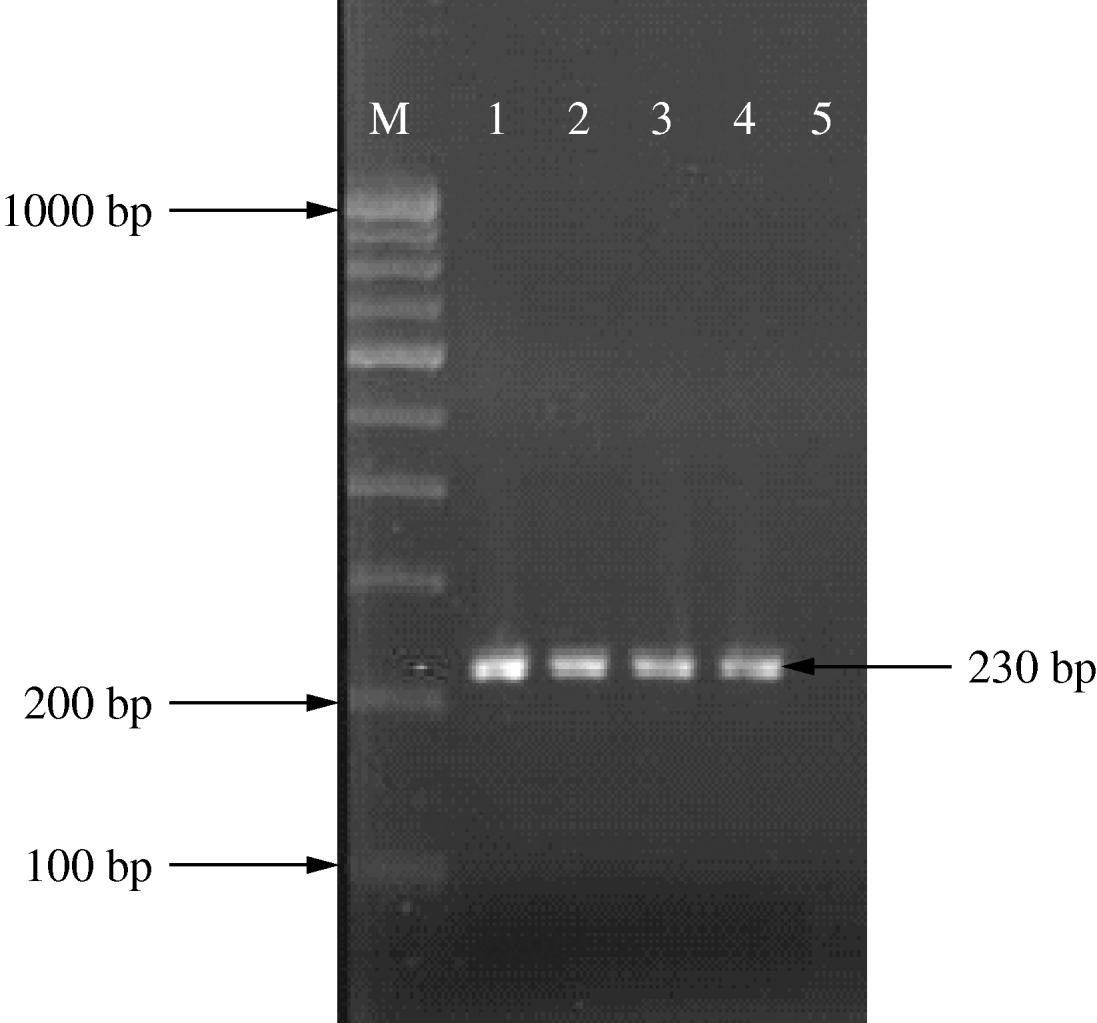
Fig. 1. PCR amplification of the CrgA gene. Lane 1, positive control; lanes 2–4, N. meningitidis-positive samples; lane 5, negative control.
RESULTS
In the current study the hospital admitted 380 suspected cases of meningococcal disease that were admitted to the meningococcal emergency isolation unit opened in the hospital during the outbreak period, only 32 were confirmed and 23 probable (55 total) according to WHO criteria. Thirty-three patients had meningitis, 14 meningococcaemia and eight had both manifestations. Eight patients, five adults and three children, died. There was a strong male preponderance with 48 males and seven females. The age distribution was three (5·5%) patients aged ⩽5 years, 18 (32·7%) 6–14 years, 25 (45·5%) 15–29 years, six (10·9%) 30–44 years and three (5·5%) ⩾45 years.
Gram stain of petechial smear gave the highest positive yield and blood culture by far the lowest (Table 1). N. meningitidis (serogroup A) was isolated in all 26 cultures (23 from CSF, three from blood). These were confirmed by serotyping and re-confirmed by CrgA PCR (Fig. 1).
Table 1. Laboratory diagnostic tests pertaining to the diagnosis of Neisseria meningitidis in patients with probable/confirmed disease (n=55)
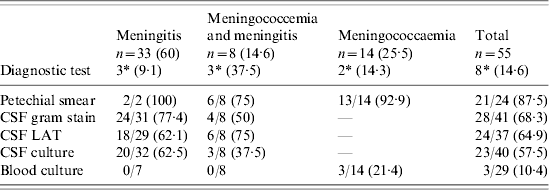
LAT, Latex agglutination test.
Figures in parentheses represent percentages.
* Mortality.
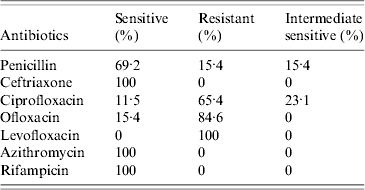
All 26 isolates were susceptible to cefotaxime, azithromycin and rifampicin. Four strains were of intermediate sensitivity to rifampicin. Susceptibility to penicillin G was 69·2%. Minimum inhibitory concentration (MIC)90 for penicillin was 1·5 μg/ml, which was within the resistant range and MIC50 was 0·032 μg/ml. Ceftriaxone also had a high MIC90 at 0·125 μg/ml upper limit of sensitivity range. MIC90 for ciprofloxacin and levofloxacin was 0·19 μg/ml and ofloxacin 0·5 μg/ml, all in the resistant range and MIC50 for all quinolones was 0·125 μg/ml. MIC50 for rifampicin was 0·064 μg/ml and MIC90 was 0·5 μg/ml. MIC90 for azithromycin was 0·25 μg/ml. Resistance to the quinolone group of antimicrobials was very high with 100% resistance observed for levofloxacin, 84·6% for ofloxacin and 65·4% for ciprofloxacin. Thirteen strains (50%) were multidrug resistant (Table 2).
Table 2. Summary of antibiotic resistance pattern of isolates
DISCUSSION
The epidemic rate for meningococcal disease in Western countries is <1–3/100 000 and 10–25/100 000 in developing countries [Reference Sachdeva13]. The outbreak in Delhi was caused by serogroup A. Five of the isolates from this hospital were subjected to PFGE and were compared to four isolates from other institutions during this outbreak; all of them belonged to a single clone. The chromosomal DNA digested with SpeI enzyme was separated by clamped homogeneous electric fields PFGE (Bio-Rad). Neisseria multi-locus sequence typing (MLST) analysis during this outbreak was performed with the MLST database showing that these isolates were similar to the outbreak strains from Dhaka, Bangladesh (2002), and Nigeria (2003) [Reference Singhal6].
Meningococcal disease remains an important cause of morbidity and mortality. An effective approach to patients with acute meningococcal disease requires recognition of the clinical syndrome and is one of the microbiological emergencies that require the laboratory to respond immediately [Reference Tunkel14]. The mortality rate of fulminant meningococcal disease varies from 10–80% [Reference Van Duren, Brandtzaeg and Van Dermeer15], which is due to diversity in the natural course of the disease, delay in diagnosis and quality of medical treatment in the hours before arrival of the patient at the tertiary-care centre. In this hospital, a mortality rate of 12·8% was observed during the outbreak. Although the conventional view of meningitis being the most common manifestation of meningococcal infections is still theoretically true, this study showed that a large proportion (40%) of cases presented with meningococcaemia with or without meningitis [Reference Periappuram, Taylor and Keane16]. This is of particular concern as meningococcaemia has a worse outcome than meningitis [Reference Lodder17].
During the outbreak there were several suspected cases of meningococcal disease which could not be confirmed or be included as probable since the laboratory tests were negative. This was probably due to prior antibiotic intake, delay in processing of samples with, inadequate collection and transport of the sample material, etc. The age group most affected were young adults and there was a male predilection [3, Reference Manchanda, Gupta and Bhalla5]. Maximum mortality (62%) was in the 15–30 years age group [Reference Raja2, Reference Singhal6].
From the laboratory perspective the samples to be handled as priority are CSF in meningitis and blood in meningococcaemia. Petechial smears may resemble viral rashes; they also do not appear in about 20% of cases. However, once haemorrhagic rash appears it is claimed to be the best indicator for making a rapid diagnosis of meningococcal disease [Reference Periappuram, Taylor and Keane16]. Haemorrhagic rash was observed in 86·9% of our patients with meningococcaemia. Microscopy of petechiae is a simple, cheap and rapid screening test with a reliability ranging from 50–90% [Reference Van Duren, Brandtzaeg and Van Dermeer15, Reference Reger and Kunz18]. In our study, the positivity rate of microscopy of petechial skin smear was 86·9% and that of CSF Gram smear 64·1%. It is known that the use of cytospin for CSF can make microscopy more beneficial [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Van Duren, Brandtzaeg and Van Dermeer15].
A positive LAT was used as a criterion for confirming a case in our outbreak according to WHO criteria. However, sensitivity of LAT is variable and ranges from <50% to 93% [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Reger and Kunz18, Reference Erdem19]. Studies which encountered low sensitivity and specificity in the LAT have attributed this to cross-reaction with non-pathogenic Neisseria spp., enteric bacteria, Bacillus spp., E. coli, etc. False-positive LAT has also been observed sometimes due to cross-reaction with povidone iodine and in patients with dermoid, a benign tumour of the skin [Reference Reger and Kunz18–Reference D'Amato, Hochstein and Fay20]. In addition LAT reagents, which are imported, are expensive (a single test costs about Rupees 900/-) and not always readily available. In our study, LAT results in CSF were positive in 63·4% of samples. One false-positive result was detected in CSF, where the culture grew E. coli. The results of LAT in serum are more ambiguous and are not recommended in diagnosis of meningococcal disease [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Tunkel14].
Culture remains the gold standard for diagnosis of this disease; the positivity of culture method varies from 30% to 85% [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Olcen21]. Meningococci are extremely sensitive to adverse environmental conditions [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1], the test results can be affected by any delay in transportation, poor handling of specimens and prior antibiotic therapy [Reference Pollard22]. In our outbreak N. meningitidis was isolated from 37·7% samples. Cultures from skin biopsies have been shown to retain their positivity despite prior antibiotic intake [Reference Periappuram, Taylor and Keane16].
PCR has evolved as a rapid molecular tool and has the advantage of retaining positivity even after antibiotic therapy or low yield of bacteria. PCR has worked with samples of CSF, blood and biopsies [Reference Pollard22, Reference Carrol23]. Several workers have attempted identification of the bacterium and simultaneous serogroup characterization of the meningococcus from the sample/strain isolated [Reference Taha10–Reference Borrow12]. In our study, PCR was used to confirm the serogroup of meningococci isolated. PCR can detect as low as 1–10 bacteria/ml of sample, which is well exemplified by real-time PCR where even one bacterium can be detected [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1].
Antimicrobial susceptibility testing of N. meningitidis is tedious, as the organism is fragile and difficult to maintain [24]. E strip MIC testing is recommended as a screening tool for clinical reporting of sensitivity with agar dilution and micro broth susceptibility being performed to confirm results [Reference Janda, Knapp, Murray, Baron, Jorgensen, Pfaller and Yolken1, Reference Vazquez8]. Studies of susceptibility testing of meningococci have not been forthcoming from India [Reference Manchanda, Gupta and Bhalla5, Reference Singhal6, 25–Reference Annapurna, Bhave and Mathur28]. In most of these reports few isolates of the bacterium have been studied and the clinical response to therapy was considered as adequate for detecting susceptibility. Penicillin has traditionally been recognized as the antibiotic of choice but in recent years reports of resistance of meningococci to penicillin have been noted, although the frequency with which such resistant isolates are found varies widely [Reference Tapsall29–Reference Jorgensen, Crawford and Fiebelkorn32].
Workers have observed in the past that a rise in MIC to penicillin is associated with higher mortality [Reference Annapurna, Bhave and Mathur28, Reference Tapsall29]. This was also observed in our study. All eight patients who died had isolates which were resistant to penicillin or high MIC. These patients also had higher MIC to ceftriaxone. In this outbreak all cases were treated with parenteral ceftriaxone. Thus far, resistance to third-generation cephalosporins is rare [Reference Manchanda, Gupta and Bhalla5, Reference Singhal6].
Resistance in N. meningitidis has been sequentially acquired by the agents used for chemoprophylaxis. Sulphonamides and tetracyclines have been rendered totally ineffective and can no longer be used [Reference Shultz, Tapsall and White33]. Rifampicin-resistant meningococci have also been encountered [Reference Rainbow34]. The quinolones to which a high degree of resistance was revealed in our study and in reports from India during this period [Reference Singhal6] are being advocated extensively for prophylactic purposes due to their oral route of administration, low cost, and easy availability [Reference Shultz, Tapsall and White33]. Resistance of meningococcus to quinolones has been documented [Reference Shultz, Tapsall and White33, Reference Capoor35, Reference Mehta and Goyal36]. The emergence of quinolone resistance in serogroup A is unique to India. This is a cause for concern as it can lead to epidemics/pandemics, which have serious implications. Quinolones have been used extensively in India for therapy of enteric fever as Salmonella had become resistant to chloramphenicol and other first-line agents. The beginning of this millennium marked an increase in nalidixic acid-resistant isolates (hallmark of the therapeutic failure of quinolones) of Salmonella Typhi and Paratyphi which are now showing frank resistance to ciprofloxacin [Reference Capoor35]. N. gonorrhoeae might have contributed to resistance in this meningococci as quinolone resistance is well documented in them [Reference Singhal6]. Resistance in these genera has been associated with the increased misuse of quinolones with over-prescription, sale of spurious drugs, etc. [Reference Capoor35]. Although failure of chemoprophylaxis to quinolones and emergence of secondary cases could not be demonstrated in the present study this aspect needs further research. Continued surveillance of resistance of meningococci to quinolones will aid in formulating guidelines at the national level. The use of ciprofloxacin as an agent of chemoprophylaxis certainly needs revisiting.
As N. meningitidis infections can rapidly develop into epidemic proportions it needs rapid as well as continuous vigilance. The high rate of mortality and serious complications associated with the disease can be prevented only by early recognition, and prompt and appropriate therapy. Thus the importance of a simple and effective diagnostic tool such as the Gram stain in the diagnosis cannot be overemphasized. Amongst the diagnostic parameters, speed of diagnosis could be achieved with smear and PCR. As the occurrence of petechiae is rare, petechial smear cannot be the ideal test. CSF smear prepared using a cytospin might be the answer for a rapid effective test. Availability and reliability of PCR and other molecular tests are a serious limitation to their application in routine diagnosis. Finally, the gold standard of culture, although invariably performed has a relatively poor sensitivity. The ‘ideal’ test for diagnosis is perhaps a combination of tests. Antimicrobial susceptibility is vital in treating the individual and crucial in guiding approach to chemoprophylaxis. The current outbreak was probably effectively controlled by the timely intervention of the Government with isolation, therapy, chemoprophylaxis and immunoprophylaxis as was necessary.
DECLARATION OF INTEREST
None.







