INTRODUCTION
Brucellosis is a highly contagious epizoonosis caused by a group of microorganisms belonging to the genus Brucella [Reference Batashev1]. Brucellae were identified for the first time by David Bruce in 1887, and named after him. These microorganisms are coccobacilli, Gram-negative, non-spore-forming, non-motile, facultative anaerobes; an important characteristic of Brucella is that they act as facultative intracellular parasites. Nine different species of Brucella are currently known: B. melitensis, B. abortus, B. suis, B. canis, B. neotomae, B. ovis, B. ceti, B. pinnipedialis, and B. microti. The first three are the main causes of disease in humans. Humans are just one of the possible hosts, in addition to other mammals including bovines, ovines, caprids, camelids, canids and some rodents, with different specificity and spread depending on Brucella spp. In lactiferous animals infection tends to become chronic, with recurring abortions, while the concentration of microorganisms remains high in faeces, urine, placenta, aborted products of conception, and milk. Humans become infected principally by ingesting unpasteurized milk and fresh cheese, but it is also an occupational disease acquired through prolonged contact with infected animals, particularly if workers assist with delivery. In these cases, infection occurs through skin lesions, and, more rarely by inhalation of aerosols. The pathogens spread via haematogenous dissemination to the organs of the reticuloendothelial system: liver, spleen, lymph nodes and bone marrow. Metastatic focalizations are also very important, the most serious localizations occurring in the nervous system: meningitis, encephalitis and nevritis, and, more rarely, endocarditis, pyelonephritis, pneumonias and thyroiditis.
Osteoarticular localizations are also common, principally sacroiliitis or lumbar spondylitis. In 1929 the pathologist Pedro-Pons described [Reference Pedro-Pons and Farreras2] epiphysitis of the anterosuperior angle of lumbar vertebrae as a characteristic sign of brucellosis. Its radiological expression (the ‘so-called’ Sign of Pedro-Pons) is characterized by a selective sclerosis in the anterosuperior angle, considered to be diagnostic of brucellosis. Although epiphysitis of the superior vertebral angle is frequently associated with osteolytic activity in haematogenous vertebral osteomyelitis [Reference Uhlinger3, Reference Waldvogel, Medoff and Swartz4], several distinctive features of these lesions indicate that they were caused by brucellosis, because of the particular arterial circulation patterns of the vertebrae [Reference Mikolich, Boyce, Mandell, Douglas and Benner5]. Of particular significance are strong, frequent sclerotic reactions below the osteolytic areas with non-involvement of the small posterior intervertebral articulations [Reference Pedro-Pons and Farreras2, Reference Spink6]. The sclerotic reactions are primarily due to endosteal activation, with thickening of the trabecular network produced by an increase in both the volume and the number of trabeculae which results in a reduction of the marrow spaces. There is also periosteal activation due to the involvement of the periosteum; the anterior faces of the vertebrae are marked by chronic inflammation. Finally, there are ossifications at the centre of the anterior faces of the affected vertebral bodies; these form as a result of the osseous metaplasia of the anterior longitudinal vertebral ligament, which is also involved in the progression of the disease. These bone lesions, arising from varying pathogenetic origins (endosteal, periosteal, metaplastic), combine to produce a sclerotic hemi-ring, known as the brucellar sclerotic vertebral hemi-ring, which can be quite dense and surrounds the area of osteolysis in the anterosuperior somatic angle from below and behind. The vertebral lesions are also restricted to characteristic sections of the spinal column (the dorsolumbar tract), the vertebrae (the anterosuperior angle) and the upper vertebral plate (the region below the impression of the annulus fibrosus). Vertebral lesions may be associated with rib alterations that are probably attributable to micronodular pleurisy that commonly strikes brucellosis victims.
However, differential diagnosis must consider other infectious diseases including tuberculous and staphylococcic spondylitis, which in modern humans occasionally cause anterior epiphysitis of the vertebral body [Reference Wiley and Trueta7]. Nevertheless, these diseases seem less likely to produce the complex set of alterations which we observed. For instance, unlike early brucellosis that frequently affects the lumbar column (particularly L4 and L5), these other diseases are usually not circumscribed and frequently involve other vertebrae. Vertebral brucellosis can be distinguished from tuberculosis because the former does not usually cause paravertebral abscesses, vertebral collapse, or gibbus formation and tends to present destructive and reparative process simultaneously, whereas tuberculosis is largely destructive.
Brucellar epiphysitis is also very different from Scheuermann's disease, which affects more than one vertebral element, usually the dorsal segment.
Brucellosis, despite important and successful eradication campaigns, remains today the commonest epizoonosis, present on all continents and affecting half a million new people every year [Reference Pappas8]. The Mediterranean countries of Europe, northern and eastern Africa, Near East countries, India, Central Asia, Mexico and Central and South America are especially affected, but travellers also bring the disease to non-endemic countries [Reference Memish and Balkhy9].
PALEOPATHOLOGY
The human brucelloses are epizoonoses and their presence in humans usually results from contact with domestic vectors, such as cattle, horses, pigs, goats, sheep, and dogs. In general the presence in the pathocenosis in ancient human communities is correlated with the diffusion of domestication of animals. Nevertheless, in 2009 our research team described the first case of a possible infectious pathology in an australopith (Australopithecus africanus Stw 431) from the late Pliocene hominin site of Sterkfontein (South Africa) [Reference D'Anastasio10]. The skeleton belonged to an adult individual, probably male, and is comprised of 18 mostly incomplete bones derived from the axial skeleton, pectoral girdle, upper limb, and the pelvic girdle. The vertebral column consists of nine consecutive thoracolumbar vertebrae, T9 to L5. The lumbar vertebra L5 of Stw 431 has a destructive focus on the superior-anterior margin of the body, with clear signs of active bone reaction (Fig. 1). Scanning electron microscopy (SEM) analysis of the trabeculae surrounding the walls of the lytic lesion revealed sheets of new bone formation and Howship's lacunae due to the osteoclastic and osteoblastic cell activity (demonstrating that the bone alteration occurred in vitam). The lateral radiograph showed the destructive lesion of the anterior vertebral body and a sclerosis limited to the area of destruction (more evident below the lesion) consistent with the Sign of Pedro-Pons [Reference Pedro-Pons and Farreras2].

Fig. 1. Lumbar vertebra L5 of Australopithecus africanus Stw 431 (Pliocene, Sterkfontein, South Africa) showing possible brucellar epiphysitis of the anterosuperior angle. (Courtesy of D'Anastasio R., Section of Anthropology, State University ‘G. d'Annunzio’, Chieti, Italy.)
The lumbar vertebra L4 of Stw 431 presented granulomatous tissue in a clearly delimited region of the anterior rim of the body. The SEM images revealed bone destruction mediated by osteoclasts, whose activity may have been stimulated by the pathogenic organisms. The radiographs of the sample also revealed sclerotic repair of the lesion.
The macroscopic, microscopic and radiological appearance of the lytic lesions of the lumbar vertebrae L4 and L5 demonstrated their pathological nature and were consistent with all the skeletal pathognomic characteristics of brucellosis.
The hypothesis of brucellosis in a 2·3–2·5 million-year-old hominid possibly related to the lineage leading to Homo sapiens, has potentially important, wide-ranging implications. The presence of brucellosis is most often associated with the consumption of animal proteins, and Australopithecus africanus could have contracted brucellosis by eating infected parturient discharges, fetal membranes, or the meat of young antelopes and other Ungulata [Reference Godfroid11].
Meat eating is well documented among baboons and chimpanzees and therefore a similar dietary behaviour in australopiths or first species of the genus Homo seems likely [Reference Pfeiffer12]. The consumption of meat increased in our more recent ancestors (i.e. H. neanderthalensis), and they, too, occasionally could have been exposed to brucellar infection. The description of a novel Brucella isolated in association with two cases of stillbirth in non-human primates [Reference Schlabritz-Loutsevitch13] opens the possibility of the existence of unknown brucellar species in the past. Yet, up to now, there is no paleopathological evidences of brucellosis in the middle and upper Paleolithic.
The next early cases of brucellosis date to the Early Bronze Age (Table 1). Brothwell [Reference Brothwell and Kenyon14] reports a case of possible brucellosis in an incomplete specimen from Jericho that presents bone inflammation of the lumbar spine and the two fibulae.
Table 1. Paleopidemiological data on brucellosis
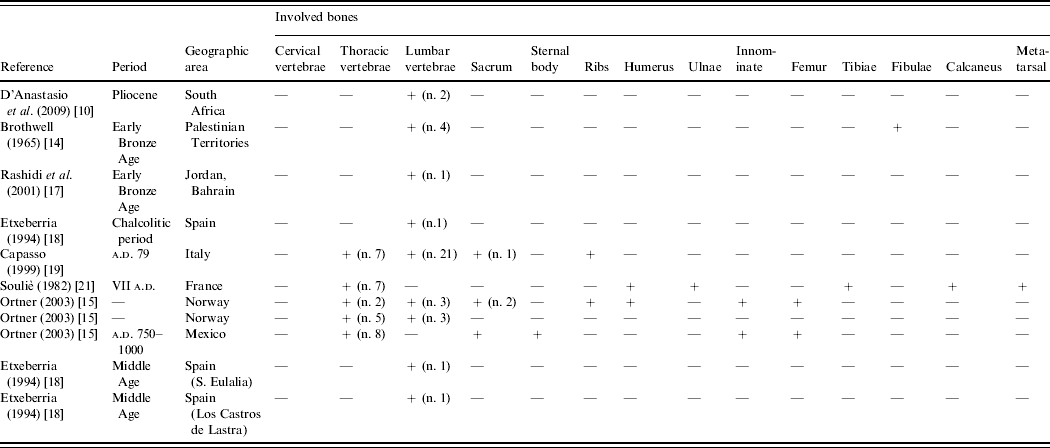
Another case of brucellosis occurs in the spine of an adult skeletal individual from the archaeological site of Bab edh-Dhrà (Jordan), dated to about 3100 b.c. [Reference Ortner15]. One of the lumbar vertebrae shows an excavation of the anterosuperior body with a large osteophyte extending in the sagittal axis and trabecular sclerosis adjacent to the lesion, demonstrated by the radiological picture.
Ortner & Frohlich [Reference Ortner and Frohlich16] and Rashidi et al. [Reference Rashidi17] have diagnosed brucellosis in human remains from other Bronze Age archaeological sites in Jordan and Bahrain.
In Europe a case of epiphysitis with erosion of the anterior vertebral rim and radiological sclerosis in an adult male from the Calcolitic period was found in Spain's Basque country and is believed to represent an early case of brucellosis [Reference Etxeberria18].
Capasso [Reference Capasso19] described vertebral lesions observed in the skeletons of adults who fled to the ancient beach at Herculaneum during the eruption of Mount Vesuvius, where they were buried alive. In all cases the lesions present as osteolysis of the superior vertebral angle (Fig. 2a). Vertebral lesions that correlate with melitococcic spondylitis were found in 16 subjects (about 17·4% of the adults) and are more common in men than in women (12 adult males and 4 adult females, a M/F ratio of 3/1). The high frequency of the disease is supported by historic evidence; indeed, written sources (Varro, R.R., II, 5,4; Cicero, De Natura Deorum, II, 159; Pliny the Elder, N.H., VIII, 180) mention that the Romans made considerable use of milk from sheep and goats. It was consumed directly, without cooking or other forms of treatment, and also as milk derivates, including ovine-milk yogurt and an impressive variety of fresh cheeses. The important role of ovine milk in the Roman diet could explain the great frequency of brucellosis at that time. The data from the Herculaneum population provides the first evidence in favour of a milk-related source for the disease. Three years later Capasso [Reference Capasso20] had the opportunity to analyse a single carbonized cheese found in Herculaneum and stored in the Boscoreale Museum (Naples, Central Italy) (sample no. 1334) (Fig. 2c). Although the high temperature and the long interval spent by the organic remains under volcanic mud precludes the possibility of identifying the bacteria through molecular methods, the SEM analysis of the cheese revealed the presence of two types of bacterial remains. The first one is a bacillar-like type, arranged in chains, whose morphology, dimensions, and colony arrangement are consistent with a member of the family Lactobacillae. The second one is a cocco-like bacterium (Fig. 2d) that, both in terms of morphology and dimensions, seems to be consistent with Brucella, although its aspect also matches that of streptococci. Despite the uncertain identification of the bacterial remains, the analysis of the carbonized cheese revealed, for the first time, a quantity of bacteria that indicates a category of food as a possible vehicle of pathogens in human epizoonoses in ancient times. The paleopathological study of the skeletons from Herculaneum suggests that brucellosis would almost certainly have been endemic in Roman society.

Fig. 2. Ancient remains from Herculaneum (a.d. 79, Italy). Fourth lumbar vertebra of subject E139, showing lithic lesion of the anterosuperior corner (a); X-ray documentation of the ‘so-called’ Pedro-Pons sign, which is the radiographic appearance of under-lithic bone sclerosis typical of brucellar vertebral epiphysitis (b). Macroscopic appearance of the carbonized cheese (c); monomorphic and monodimensional cocci (~0·8 μm) showing large holes with invaginated borders (scanning electron microscope ×25 000) (d). (Courtesy of Capasso L., Section of Anthropology, State University ‘G. d'Annunzio’, Chieti, Italy.)
Continuing our timeline, the next case of brucellar infection is reported by Souliè [Reference Souliè21] who diagnosed the disease in a skeleton excavated at Raucourt (France), dated to the last half of the seventh century. The specimen belonged to a 35-year-old female and shows infectious lesions in the thoracic spine, left calcaneus and fifth metatarsal, and ankylosis in the right elbow joint.
Etxebarria [Reference Etxeberria18] reports two cases of brucellosis in young adult males of the later Middle Ages. The first skeleton came from the necropolis of Los Castros de Lastra (Alava, Spain); it shows an epiphysitis of the anterior and superior rim of vertebra L3 characterized by a granulomatous aspect and a marginal sclerosis, visible by radiography. The second one, from the necropolis of Santa Eulalia (Alava, Spain) presents an epiphysitis of the anterior and posterior angle of vertebra L4 with sclerosis limited to the area of injury.
To a more recent period belong cases of vertebral spondylitis from the archaeological site of Tysfjord (Nordland, Norway) that Ortner [Reference Ortner15] diagnosed as brucellosis. One is a young male aged about 19 years at the time of death; he is thought to be associated with the Lapp people, known for their close association as herders of caribou. His spine exhibits multifocal lytic lesions with minimal sclerosis in their margins, from mid-thoracic through lumbar vertebrae. The second case is a female Lapp skeleton aged 20–25 years at death showing lytic lesions and erosions in several bones (left humerus, several thoracic and lumbar vertebrae, sacroiliac joints, and left femur).
Ortner [Reference Ortner15] reports only one case of possible brucellosis from the Americas, from an historic archaeological site in Merida (Mexico). The skeleton is from a female aged about 40 years at the time at death. Its clinical picture is complex: there are multiple destructive foci in the thoracic vertebrae, the sacroiliac joint, the sternal body and the left distal femur. After discussing the differential diagnosis with fungus infection, echinococcosis, and metastatic carcinoma, the authors suggest brucellosis as the most probable aetiology (principally based on the lesions on the sacroiliac joint).
PALEOEPIDEMIOLOGY
On the basis of the cases reported above, it is clear that brucellosis receives little attention in the literature on paleopathology, perhaps as a consequence of the problematic differential diagnoses with other infectious diseases such as tuberculosis and staphylococcic spondylitis. Nevertheless the paleopathological data suggest some considerations.
Brucellosis in humans is currently comparatively rare in developed countries [Reference Norden, Gillespie and Nade22]. Most of the recent reports have come from areas such as North Africa and the Middle East. The disease in humans occurs as a chronic infection of the lungs and other organs, and, in a number of cases, the skeleton becomes infected by the haematogenous route [Reference Spink6]. Skeletal involvement varies from 2% to 70% of the cases [Reference Jaffe23] and 20% to 80% of patients with brucellosis experience osteoarticular symptoms [Reference Mikolich, Boyce, Mandell, Douglas and Benner5]. Adult males are affected much more frequently than females [Reference Glasgow24]. The most common skeletal lesion occurs in the spine or the sacroiliac joint [Reference Madkour, Sharif and Madkour25, Reference Rajapakse26]. Ganado & Craig [Reference Ganado and Craig27] observed 130 instances of spondylitis in 6300 patients with brucellosis. Long bones are rarely involved [Reference Ortner15]. Kelly et al. [Reference Kelly28] observed in 36 cases the following distribution: spine 47·2%, humerus 8·3%, femur 5·6%, ilium 2·8%, hand 2·8%, foot 2·8%. The spinal lesions involve the vertebral bodies, especially of the lower thoracic, lumbar, and lumbosacral areas [Reference Madkour, Sharif and Madkour25, Reference Lowbeer29, Reference Lowbeer30]. The early skeletal signs of brucellar diseases are characterized by epiphysitis of the anterosuperior angle of the lumbar vertebrae, radiographically showing a selective sclerosis in the anterosuperior angle of the vertebra; this radiographic expression is known as the Sign of Pedro-Pons and represents a pathognomonic aspect of the early stage of brucellar disease in ancient human remains [Reference Mousa31–Reference Geyik37].
From a paleoepidemiological standpoint brucellar bone lesions are more frequent in males than in females, with the M/F ratio being 2:3 and they are generally seen in adult individuals.
The osteoarticular localizations of brucellar lesions in ancient human remains reflect the same pattern observed in modern cases, with the following distribution: spine 45·2%, sacroiliac joints 16·1%, lower long bones 12·9%, upper long bones 9·7%, ribs 6·5%, foot 6·4%, sternal body 3·2%. The vertebral region most frequently affected is that including the lower dorsal and upper lumbar vertebrae, and most of the affected vertebrae show erosion of the upper anterior somatic corner (so-called anterior vertebral epiphysitis) [Reference Di Rienzo38], with or without limited extension to the central part of the vertebral plate, and osteosclerosis under the angular osteolysis (the Sign of Pedro-Pons) [Reference Pedro-Pons and Farreras2].
The distribution and frequency of bone lesions in ancient human remains ascribed to brucellosis present some differences from those ascribed to other possible causes. The bones most frequently affected by tuberculosis are the spine, tarsal and metatarsal, ribs, sternum and long bones; in other words the skeletal areas of haemopoietic (red) marrow, which has high circulatory and metabolic rates. In vertebral tuberculosis, the most common site is the first lumbar vertebra, with the frequency decreasing with the distance on either side of this site. Regarding to the distribution of skeletal tuberculosis in joints, the knee is most frequently involved, followed by hip and elbow [Reference Waldvogel, Medoff and Swartz4]. Haematogenous osteomyelitis caused by other infectious organisms (i.e. Staphylococcus, Streptococcus, Haemophilus) more often affect long bones, ribs and spine. Joints, spine and post-cranial bones may be affected by fungal infections, but they actually contribute a very small percentage of bone lesions (with the exception of coccidioidomycosis). The skeletal lesions are mainly lytic with little if any perifocal bone reaction, similar to those of neoplastic diseases and tuberculosis [Reference Aufderheide and Rodriguez Martin39].
The most ancient cases of brucellosis in modern humans date back to the Early Bronze Age, in skeletal remains from archaeological sites of Jordan, Bahrain and the Palestinian Territories. However, it is important to note a much earlier case of possible brucellosis in a fossil skeleton of the Pliocene hominin Australopithecus africanus, ancestor of modern humans [Reference D'Anastasio10].
According to the paleopathological data, the frequency of brucellosis seems to increase during the Roman period and the Middle Ages, although its distribution seems limited to the European continent.
The only paleopathological case of brucellosis reported from the Americas came from the archaeological site of Merida in Mexico.
CONCLUSION
Brucellosis is a worldwide disease. Although it has been eradicated in some countries, it continues to be an important disease in many farming areas in the Mediterranean region (both in Europe and Africa) where it is also known as Malta fever, as well as in other agricultural regions located in New Zealand, Asia, Central America and Mexico, and South America.
Brucellosis is an ancient disease; the first possible cases were reported in skeletal individuals dated to the Middle Bronze Age from archaeological sites of Jordan, Bahrain and the Palestinian Territories. It is interesting to note that this geographic area is the original centre of the domestication of sheep and goats [Reference Clutton-Brock40], and the first cases of brucellosis in modern humans could be linked to permanent contact with these mammals and to the ingestion of milk and dietary products from infected animals.
Nevertheless our research group diagnosed vertebral brucellosis in the partial skeleton of the late Pliocene Australopithecus africanus Stw 431 from Sterkfontein, South Africa [Reference D'Anastasio10], demonstrating that this infectious disease occasionally could have affected our direct ancestors some 2·3–2·5 million years ago.
The presence of brucellosis seems to increase in Europe during the next historical periods (Roman and the Middle Ages), perhaps following the diffusion and habit of ovine rearing.
The examination of the skeletal population dating to a.d. 79 uncovered during the excavation of the ancient beach of Herculaneum revealed that about 17·4% of adults suffered from brucellar spondylitis. The study suggests that brucellosis would almost certainly have been endemic in Roman society. Moreover the identification of bacterial remains in carbonized cheese from the same archaeological site, together with the historical data, provide the first indication of a possible association between brucellar bone lesions and the consumption of ovine milk, which served as an important source of epizoonosis in the Roman population.
From to the New World, the only report comes from Mexico.
The ancient geographic distribution of brucellosis follows the same pattern observed today. The great majority of paleopathological cases involve adult skeletal individuals, with males more frequently affected than females.
From an anatomic standpoint, the distribution of the skeletal lesions mirrors the pattern observed in modern clinical cases. Vertebrae are most commonly involved, especially the lumbar vertebral bodies, and most of the affected vertebrae show the erosion of the upper anterior somatic corner. Long bones are affected much less frequently than the spine, while flat bones are least often infected. The sacroiliac joints are the most commonly affected joints.
Because skeletal involvement is relatively common, it seems likely that additional skeletal evidence of brucellosis might be found in ancient human remains. However, brucellosis receives little attention in the literature on paleopathology, perhaps because differentiating skeletal lesions of this diseases from other diseases that can affect the skeleton is a challenging exercise. This difficulty might explain the limited number of cases reported in the literature, but we hope that future studies will bring a more complete paleoepidemiological picture of brucellosis.
DECLARATION OF INTEREST
None.







