INTRODUCTION
Campylobacter jejuni is the leading cause of bacterial foodborne gastroenteritis throughout the world [Reference Blaser1–Reference Wit4]. It is a Gram-negative, motile microorganism, which is primarily micro-aerophilic. Remarkably, this pathogen grows within a short temperature range, being unable to multiply at temperatures above 45°C or below 30°C. At conditions where C. jejuni cannot grow, such as low temperature or in spent medium, it loses its culturability. It is crucial to know whether non-culturable C. jejuni can cause an infection, as in practice the exposure is often measured as the number of culturable C. jejuni in a product.
In the literature many contradictory articles on the infectivity of non-culturable C. jejuni can be found. Some authors showed that non-culturable C. jejuni were not infective in chicks, mice and human volunteers [Reference Beumer, Vries and Rombouts5–Reference Medema8] while others demonstrated that non-culturable C. jejuni were colonizing chicks and mice [Reference Cappelier9–Reference Stern11]. The inconsistency in the literature about the infectivity of non-culturable C. jejuni might be the result of differences in methods, conditions and strains used. A marked difference between studies is the temperature at which non-culturable C. jejuni were formed. While some authors did not find any infectivity of non-culturable C. jejuni formed at 4°C [Reference Fearnley and Newell6–Reference Medema8], in all studies reporting on non-culturable C. jejuni causing infection, the non-culturable C. jejuni were formed at 4°C [Reference Cappelier9–Reference Stern11], whereas in all experiments using higher temperatures no infectivity was found [Reference Beumer, Vries and Rombouts5, Reference Medema8]. Interestingly Hazeleger et al. [Reference Hazeleger12] showed that non-culturable C. jejuni formed at 4°C showed characteristics, including intracellular/extracellular ATP ratio and membrane fatty-acid composition, comparable to culturable C. jejuni, whereas non-culturable C. jejuni formed at 25°C were clearly different. The aim of this study was to further elucidate if non-culturable C. jejuni formed at 4°C can be infective.
The non-culturability of suspensions, in studies in which non-culturable C. jejuni were found to be infective, is often discussed. If only one or a few undetected culturable bacteria remain present, the observed infectivity might be caused by these undetected culturable bacteria instead of by the non-culturable bacteria. To avoid the misleading effect of a few culturable bacteria, in our study the infectivity of culturable C. jejuni suspensions and the infectivity of the same culturable C. jejuni suspensions supplemented with a high number of non-culturable C. jejuni, formed at 4°C, were measured. The comparison was made for different doses of culturable C. jejuni in the absence and presence of non-culturable C. jejuni, as the infectivity might be dose-dependent [Reference Friis13–Reference Mooney15].
For our study, INT-407 cells were chosen, based on various studies showing that the human cell lines Caco-2 and INT-407 mimic best the outer cell layer in the small intestines [Reference Konkel16, Reference Melo and Pechere17]. In preliminary work (data not shown) adhesion and invasion were measurable in both Caco-2 and INT-407 cells, but IL-8 secretion after exposure with C. jejuni was only detected in INT-407 cells. The INT-407 cells were used for two in vitro infectivity tests. First, the adhesion and invasion assay, which is based on the binding to and entry in host cells of C. jejuni, an important factor in the pathogenesis of C. jejuni [Reference Wooldridge and Ketley18]. Second, the IL-8 assay in which the C. jejuni-stimulated secretion of the cytokine IL-8, an early signal for the mucosal inflammatory response [Reference Eckmann, Kagnoff and Fierer19, Reference Jung20], is determined as a measure for the immune response. A disadvantage of the adhesion and invasion assay is that the outcome is measured by plate counting. If non-culturable C. jejuni do not recover their culturability during the assay, but do adhere or invade, the infectivity of non-culturable C. jejuni is underestimated. As the IL-8 assay is based on measuring an immune response, even if non-culturable C. jejuni do not recover, their effect on the infectivity is likely to be measured by the IL-8 assay. As in vitro studies have established that the invasive and adhesive ability of C. jejuni strains differ [Reference Biswas, Itoh and Sasakawa21–Reference Tay27] and, furthermore, that the C. jejuni-stimulated IL-8 secretion is strain dependent [Reference Hickey28, Reference Hickey29] four C. jejuni strains were selected. C. jejuni 70.2 and BF were chosen as for these strains the formation of infective non-culturable C. jejuni has been described by Cappelier et al. [Reference Cappelier9, Reference Cappelier and Federighi30]. C356 and 82/69 were selected for their good adherence and invasion properties found in preliminary research (data not shown).
METHODS
Culturing C. jejuni strains
Strains were stored at −70°C in brain heart infusion broth (BHI, Difco, Sparks, MD, USA) plus 30% (v/v) glycerol in cryovials. For culturing C. jejuni strains 70.2 and BF (INRA, Nantes, France; both isolated from human faeces), C356 (ID-Lelystad, The Netherlands; isolated from chicken faeces) and 82/69 (ID-Lelystad; isolated from chicken faeces, same serotype also found in human faeces), the content of one vial (0·5 ml) was thawed and put in a wide-necked Erlenmeyer flask with 50 ml BHI. The flask was incubated while shaking at 100 rpm in a custom-made incubator (NuAire, Plymouth, MN, USA) with a micro-aerobic atmosphere (10% O2, 5% CO2, 85% N2) at 37°C. After ±24 h, 0·5 ml was subcultured in 100 ml fresh BHI and incubated under the same conditions for ±16 h. These suspensions were used in the cell line assays.
Culturability
Plate counts were performed by spread plating 0·1 ml of appropriate decimal dilutions of bacterial suspensions in sterile peptone (Difco, 1 g/l) saline (Merck, Amsterdam, The Netherlands; 9 g/l NaCl) solution on Columbia agar base with 5% (v/v) defibrinated horse blood (CAB, Oxoid, Basingstoke, UK). The plates were incubated micro-aerobically at 37°C in a jar with BBL® Campypak (Becton Dickinson, Sparks, MD, USA) for 72 h.
Non-culturable C. jejuni suspensions
To obtain non-culturable C. jejuni, strains were cultured as described above. After culturing, the bacterial suspensions (±109 C. jejuni/ml) were stored aerobically without shaking at 4°C in wide-necked Erlenmeyer flasks covered with cottonwool and kitchen foil to prevent dehydration. When plate counts were below the minimal detection level (=10 c.f.u./ml), usually after 30 days, suspensions were considered to be non-culturable. For the infection assays, in which 40 μl suspension was used, this corresponds to 4×107 non-culturable C. jejuni/well and ⩽0·4 culturable C. jejuni/well.
INT-407 cell line, growth media and conditions
Human embryonic intestinal cells (INT-407) obtained from the American Type Culture Collection were maintained in minimal essential medium with Earle's salts and without glutamine (EMEM, Gibco, Life Technologies Ltd, Paisley, Scotland) supplemented with 10% heat-inactivated (30 min at 60°C) fetal bovine serum (FBS, Integro b.v., Zaandam, The Netherlands), 6 mml-glutamine (Gibco) and 50 μg/ml gentamycin (Gibco). Cells were grown routinely in 10 ml culture medium in a 75-cm2 flask (Corning Costar Europe, Badhoevedorp, The Netherlands) in a CO2 5% (v/v) incubator at 37°C. Confluent stock cultures were washed and released with 0·05% trypsin-EDTA and new stock cultures were seeded with 105 cells/ml. For the adhesion/invasion and IL-8 assays, 12-well tissue culture plates (Corning Costar Europe) were seeded with 160 000 INT-407 cells/ml per well. The plates were incubated in a CO2 5% (v/v) incubator at 37°C; the medium was changed three times a week. The plates were used 8 days after seeding.
Infectivity assays
Prior to the experiment, the medium overlaying the 8-day-old monolayers in the 12-well plates was replaced by pre-warmed EMEM, supplemented with 6 mml-glutamine. After 1 h the cultures were inoculated with 103–1010 culturable C. jejuni/well without or with 4×107 non-culturable C. jejuni of the same strain per well. Bacteria were allowed to adhere to and invade INT-407 cells for 2 h in a CO2 5% (v/v) incubator at 37°C. After this incubation the bacteria were removed by rinsing the monolayers three times with EMEM.
To study adhesion and invasion, the INT-407 cells were lysed with 1 ml 1% (v/v) Triton-X100 (Merck) in distilled water. The number of bacteria adhering to and/or invading INT-407 cells/well was determined by plating serial dilutions of the suspensions on CAB and counting the resulting colony-forming units, after 72 h incubation at 37°C under micro-aerobic conditions. Adhesion and invasion assays were performed in triplicate.
To study IL-8 secretion, 1 ml EMEM with 50 μg/ml gentamycin was added to the cells, followed by incubation for 24 h in a CO2 5% (v/v) incubator at 37°C. Subsequently, supernatants were collected and stored at −70°C to be analysed later. INT-407 cells without addition of bacteria were used as control. IL-8 concentrations were determined in triplicate using an IL-8 ELISA according to Garssen et al. [Reference Garssen31].
RESULTS
Adhesion and invasion
The number of bacteria adhering to and/or invading INT-407 cells/well after infection with increasing numbers of bacteria, in a range of 103–1010 bacteria/well, was determined for four different strains, C356, BF, 70.2, and 82/69, in the absence or presence of ±4×107 non-culturable C. jejuni of the same strain per well (Fig.).
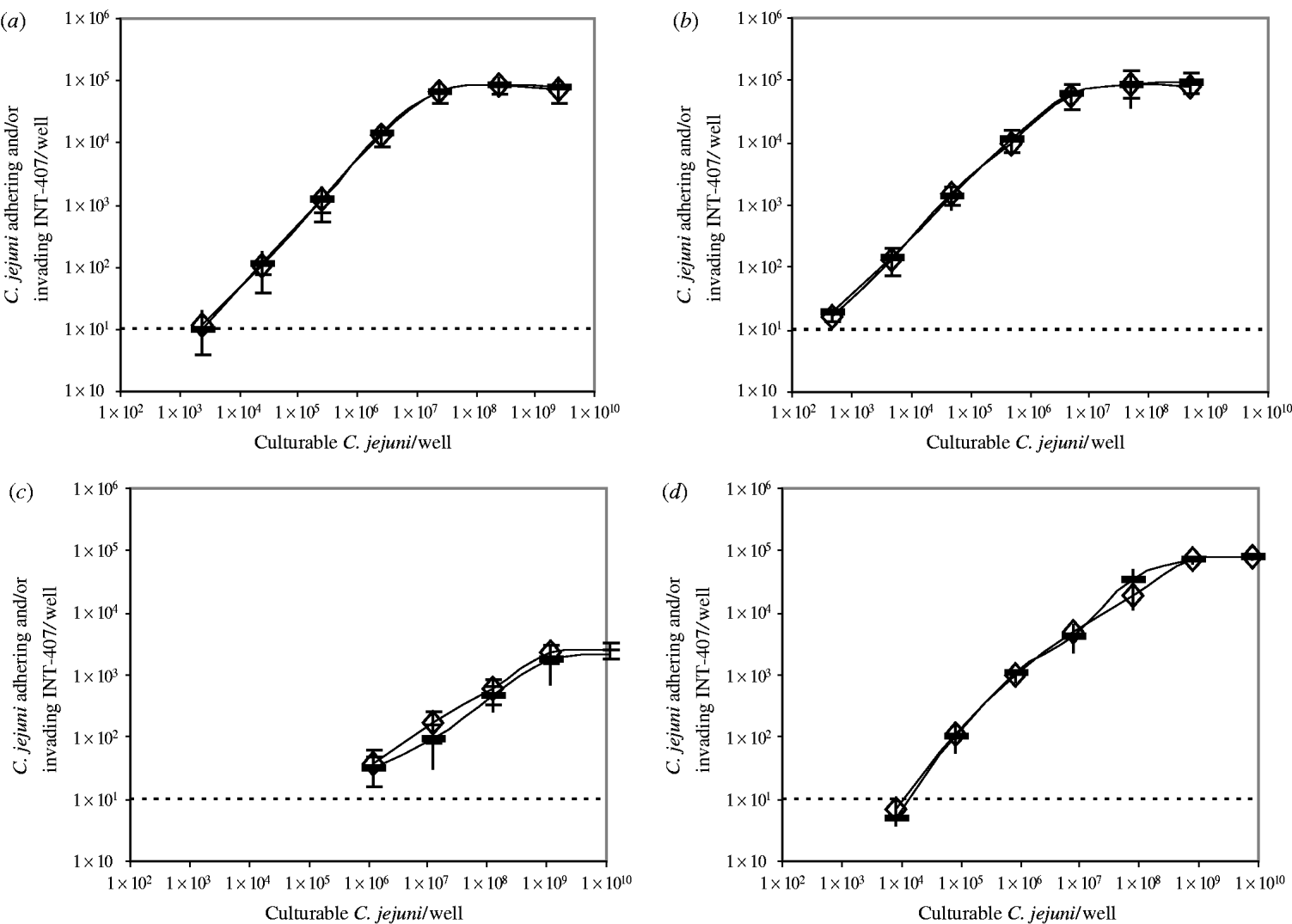
Fig. Bacteria adhering to and/or invading INT-407 cells/well after incubation with increasing numbers of culturable C. jejuni in the absence (–) or presence (◊) of ±4×107 non-culturable C. jejuni/well. (a) C. jejuni C356, (b) C. jejuni BF, (c) C. jejuni 70.2, (d) C. jejuni 82/69. The detection limit of the assay was 10 bacteria adhering to and/or invading INT-407 cells/well.
A similar trend was observed for all four strains: the number of bacteria adhering to and/or invading INT-407 cells/well increased as the number of C. jejuni increased until a maximum was reached, however, the curves differed per strain. The minimal needed number of culturable C. jejuni/well at which adhesion and invasion in the assay was measured, varied from ±5×102 for C. jejuni BF to ±1×106 for C. jejuni 70.2. The dose at which the maximal adhesion and invasion was reached, differed from ±5×106 C. jejuni BF/well to ±1×109 C. jejuni 70.2/well. The maximum number of bacteria adhering to and/or invading INT-407 cells, varied from ±8×104 C. jejuni/well for strains C356, BF and 82/69 to ±2×103 C. jejuni/well for strain 70.2.
No difference was seen between measurements in the absence or presence of non-culturable C. jejuni.
IL-8
The IL-8 secretion by INT-407 cells after infection with the four strains in the absence or presence of non-culturable C. jejuni of the same strain was measured (Table).
Table. IL-8 secretion by INT-407 cells, after incubation with culturable C. jejuni in the absence or presence of ±4×107 non-culturable C. jejuni/well
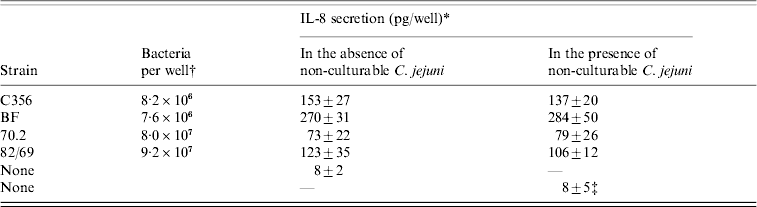
* All IL-8 determinations were performed in triplicate, results are means±standard deviation.
† As undiluted overnight cultures are used, the number of bacteria added per well differs.
‡ Cocktail (1:1:1:1) of non-culturable C. jejuni of the four strains was used.
The induced IL-8 secretion varied significantly per strain, with the lowest IL-8 secretion induced by C. jejuni 70.2 and the highest IL-8 secretion measured after infection with C. jejuni BF. For all strains, no difference was found between measurements in the absence or presence of non-culturable C. jejuni.
DISCUSSION
Many conflicting articles have been written on the existence and importance of non-culturable C. jejuni [Reference Beumer, Vries and Rombouts5–Reference Stern11]. To further elucidate if non-culturable C. jejuni can be infective, the effect of adding non-culturable C. jejuni on the infectivity in INT-407 cells was studied instead of measuring the effect of non-culturable C. jejuni on their own.
No differences in adhesion and invasion were found when non-culturable C. jejuni were added, which implies that non-culturable C. jejuni do not adhere or invade in vitro. However, to measure adhesion and invasion, non-culturable bacteria have to recover their culturability. If non-culturable C. jejuni can adhere or invade but cannot recover their culturability, measuring the adhesion and invasion would result in an underestimation of the infectivity, although the curves in the Figure would be affected. The curves all show an increase in the number of bacteria adhering to and/or invading INT-407 cells/well until a maximal is reached, as previously described by Biswas and colleagues [Reference Biswas, Itoh and Sasakawa21, Reference Biswas, Itoh and Sasakawa32, Reference Biswas, Niwa and Itoh33]. If non-culturable C. jejuni were able to adhere or invade, but not to recover their culturability, a competition with the culturable C. jejuni would be expected. This competition between culturable and non-culturable C. jejuni would result in a decrease in the measured number of bacteria adhering to and/or invading INT-407 cells/well, which would have affected the slope of the curves in the Figure. The number of bacteria adhering to and/or invading INT-407 cells/dose and the slope of the curve were not affected, again indicating that non-culturable C. jejuni cannot adhere or invade. Furthermore, the results of the IL-8 assay which are based on measuring an immune response, also strongly support the assumption that non-culturable C. jejuni are not infective, since the addition of non-culturable C. jejuni did not affect IL-8 secretion.
Our findings strongly indicate that non-culturable C. jejuni formed at 4°C are not infective in vitro, and conflict with the literature in which non-culturable C. jejuni formed at 4°C were found to be infective [Reference Cappelier9–Reference Stern11]. Cappelier et al. [Reference Cappelier9] even reported that non-culturable C. jejuni of strains, BF and 70.2, both used in this study, were infective in two animal models. The infectivity of non-culturable C. jejuni in these former studies might be addressed by the presence of few culturable C. jejuni. In two studies [Reference Cappelier9, Reference Stern11] culturability was determined by selective enrichment. As sublethally injured C. jejuni are sensitive to selective agents, selective enrichment will negatively influence the culturability [Reference Humphrey34] and the presence of a few culturable C. jejuni might be not detected. Another explanation might be the use of in vivo instead of in vitro models, although previous studies showed a correlation between the adhesion and invasion properties and IL-8 values in vitro to the infectivity in vivo [Reference Bacon35–Reference Newell and Pearson37].
Our results confirm that the adhesion and invasion and the IL-8 secretion are strain dependent. The more adhesive and invasive strains, appeared to be the ones which also induced the highest levels of IL-8 in INT-407 cells, as previously shown by Hickey et al. [Reference Hickey28, Reference Hickey29]. Next to strain-dependency, the level of adhesion and invasion was also found to be dose dependent as previously shown [Reference Friis13–Reference Mooney15]. Our data illustrate the importance of measuring the invasion and adhesion at different doses when comparing strains. For example at a high dose (>8×108 C. jejuni/well) the number of bacteria adhering to and/or invading INT-407 cells was comparable for C. jejuni BF and 82/69, while at a low dose (8×103 C. jejuni/well) the number of bacteria adhering to and/or invading INT-407 cells varied by a factor of 20. C. jejuni 70.2 is in all aspects the least infective strain: the minimal dose to measure adhesion and invasion is the highest compared to the other strains, and the maximal number of bacteria adhering to and/or invading INT-407 cells is by far the lowest. The cause of the differences in infectivity between strains has not yet been elucidated.
In conclusion, our findings indicate that non-culturable C. jejuni do not adhere or invade INT-407 cells and do not induce IL-8 secretion. Therefore, assuming that the INT-407 model is comparable to the effect in vivo, the number of culturable C. jejuni in a product is a good measure for the infection risk of a product.
DECLARATION OF INTEREST
None.






