INTRODUCTION
Since its first introduction in 2004, the mosquito species Aedes albopictus has become well established in southern France with a continuous further spread every year. Ae. albopictus is an efficient vector for several arboviruses including dengue virus (DENV), chikungunya virus (CHIKV) and to a lesser extent Zika virus (ZIKV) [Reference Paupy1]. DENV and CHIKV with the recent addition of ZIKV might be a threat for mainland France, since all prerequisites for autochthonous transmission are present in southern regions [Reference Rezza2]. Indeed, the regular introduction of these viruses through viraemic travellers returning from endemic or epidemic countries to France, where the population is mostly naive for arboviral infection, represents a risk for local transmission in vector-colonized areas during the activity period of mosquitoes (May–November). This situation has previously led to several episodes of autochthonous transmission of both CHIKV and DENV, with up to four small outbreaks in southern France recorded since 2010 [Reference La3–Reference Grandadam6]. In order to prevent transmission and dissemination of these arboviruses in areas colonized by Ae. albopictus, the French Institute for Public Health Surveillance (InVS) has implemented a preparedness and contingency plan during the activity period of mosquitoes [7]. This plan relies on both entomological and epidemiological surveillance. All clinically suspected cases must be reported to regional health authorities and notification of confirmed cases are mandatory in order to trigger epidemiological investigations and vector-control measures in locations visited by arbovirus-infected patients. The efficiency of this plan involves an increased awareness of physicians about the diagnosis and notification of suspected arboviral fever associated with a close coordination with a network of laboratories in which appropriate diagnostic tools have been implemented. We report here a 1-year experience (2015) in laboratory surveillance of human arbovirus infections at the university hospital of Montpellier, a city located in a southern France area where Ae. Albopictus is established.
MATERIAL AND METHODS
Patients and samples
During 2015, all patients who were sampled for a diagnosis of DENV and/or CHIKV infection, with subsequent addition of ZIKV were retrospectively included in the analysis. A suspected case of DENV or CHIKV infection was defined by rapid onset of fever (⩾38·5 °C) associated with at least one of the following signs: headache, myalgia, arthralgia, retro-orbital pain or cutaneous rash. A suspected case of ZIKV infection was defined by a cutaneous rash with or without fever with at least two of the following signs: conjunctival hyperhaemia, arthralgias or myalgias. Samples collected for viral investigation (serum or plasma) were sent to the laboratory with a notification form completed by the physician that recorded clinical signs, the date of symptom onset and, for travelling patients, the country visited and the return date. This document, edited by the French national surveillance authorities, was used as a patient informed consent for the anonymous research use of both samples and data. The diagnostic strategy followed in the laboratory was based on a consensual algorithm with molecular assays to detect arboviral RNA performed on samples obtained up to 7 days after symptom onset, whereas specific antibody detection was restricted to samples obtained after the fifth day following onset of clinical signs. In the last 2 months of 2015, a urine sample collected up to 10 days after the onset of clinical signs was added for ZIKV RNA detection.
Viral laboratory investigations
RNA was extracted from 200 µl samples (plasma, serum, urine) with the MagNA pure Compact apparatus running the MagNA pure compact nucleic acid isolation kit 1 (Roche Diagnostics, France). DENV and CHIKV RNA detection were performed on a LC480 apparatus (Roche Diagnostics) using the RealStar Dengue kit 2.0 and Chikungunya kit 2.0 assays (Altona Diagnostics, France), respectively. ZIKV RNA was detected using an in-house real-time RT–PCR based on primers and probes previously described [Reference Lanciotti8].
The DENV serotype was subsequently determined in DENV-positive samples through sequence analysis of a NS5 PCR fragment as described by Lanciotti et al. [Reference Lanciotti9]. Briefly, The NS5 PCR product of the F gene was purified with the QIAquick PCR purification kit (Qiagen, France) and sequenced on an ABI 310 sequencer with a fluorescent dye terminator kit (Applied Biosystems, France). Sequencing primers were the PCR primers. A phylogeny analysis was submitted to the French phylogeny website (http://www.phylogeny.fr) using the ‘one click version’ for analysis. The nucleotide sequences were aligned using MUSCLE software, cured with Gblocks and subsequent phylogeny performed with PhyML software. One hundred bootstrap datasets with random sequence addition were computed to generate consensus trees through TreeDyn.
DENV- and CHIKV-specific antibodies
Serological investigations were performed using the PanBio Dengue duo cassette for DENV IgG and IgM (Standard Diagnostics, Korea) and a commercial indirect immunofluorescent assay (EuroImmnun, Germany) for CHIKV-specific IgM and IgG. ZIKV-specific antibodies were assayed by the National Reference Center for Arboviruses (Marseilles, France), with in- house IgM antibody-capture ELISA (MAC-ELISA) and IgG indirect ELISA assays.
RESULTS
Population studied
Samples were obtained from 141 patients (121 adults, 20 children) with a suspected arboviral infection. Most patients were seen soon after the acute phase with a median delay of 7 days (interquartile range 4–16·5 days) between the onset of clinical signs and sampling. Sixty-nine (49%) patients were returning from DENV-, CHIKV- or ZIKV-endemic or epidemic areas (Table 1) and constituted the group of suspected imported cases (group I), whereas 72 (51%) patients who did not report any recent travel in areas at risk for arboviral infections constituted the group of suspected autochthonous cases (group II). The distribution of cases throughout the year is presented in Figure 1. The retrospective analysis of clinical data revealed that 49 (35%) patients did not strictly fit the suspected case definition; however, there was no significant differences regarding imported vs. autochthonous cases.
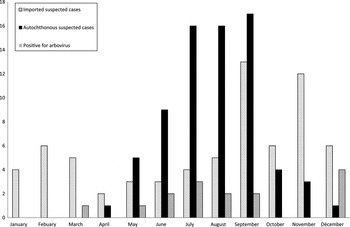
Fig. 1. Number of requests for arbovirus diagnosis during 2015.
Table 1. Origins and types of imported arboviruses

Arboviral infections
A diagnosis of acute arboviral infection was confirmed for 15 (10·6%) patients. All these cases belonged to group I comprising 69 (21·7%) patients with suspected imported cases. The viral aetiology was DENV for 13 cases plus one case for each CHIKV and ZIKV. For these cases, viral RNA was detected for 10 (76·9%) DENV-infected patients as well as in the case of CHIKV infection. The two remaining DENV infections and the ZIKV infection were diagnosed based on detection and kinetics of specific IgM and IgG antibodies. Among these confirmed cases, nine were detected during the May–November surveillance period, and were from patients infected with DENV.
For the DENV-infected samples, viral sequences were obtained for nine strains and the phylogenic analysis showed that the four serotypes of viruses were detected (data not shown). As shown in Table 1, DENV strains were mainly detected in imported cases from South America, the Caribbean, Pacific Islands and Asia whereas CHIKV and ZIKV were identified in imported cases from Tahiti and from Martinique island respectively.
Differential diagnosis
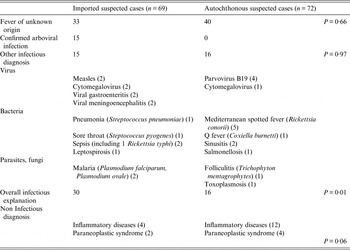
An alternative infectious aetiology could be included for 15 patients in the imported cases group and 16 patients in the autochthonous cases group (P = 0·97). A non-infectious likely origin of clinical signs (inflammatory, neoplasm) was recorded for respectively 6 and 16 patients from groups I and II, respectively (P = 0·06). The main final diagnosis was fever of unknown origins with no significant difference between the two groups (Table 2).
Table 2. Comparison of diagnosis recorded between suspected imported and suspected autochthonous cases
DISCUSSION
In 2015, the epidemiology of arboviruses was dominated by the extensive spread of ZIKV in South America and the Caribbean [Reference Musso and Gubler10]. In our series, more than one third of the suspected imported cases were patients returning from these areas; however, all but one of the confirmed cases, including those from Brazil, were infected with DENV. One ZIKV infection was detected in late 2015, in a patient returning from Martinique concomitantly with the first description of autochthonous cases in French West Indies [Reference Maria11]. This suggests that despite the epidemic spread of ZIKV, DENV remains extensively circulating. This is further illustrated by the panel of various DENV serotypes detected throughout the year from most tropical parts of the world. Imported cases reflected the worldwide arbovirus epidemiology and confirm the need for an efficient surveillance in area where the conditions of local transmission are met [Reference Rezza2]. The laboratory part of that surveillance includes the investigation for arboviral aetiologies in travellers returning from epidemic or endemic regions. In febrile travellers returning from risk area, the diagnosis of arboviral infections as well as malaria falls within normal guidelines with additional relevance during the May–November period of activity of the locally established vector Ae. albopictus in southern France. This surveillance and report of imported cases allows rapid vector-control measures in the environment of viraemic patients in order to limit the spread of the disease. Another part of the plan is to increase awareness of clinicians regarding the detection of what could be autochthonous cases. In 2015, the previous experience of a 12 autochthonous CHIKV case outbreak in Montpellier in 2014 [Reference Maria11] had probably reinforced clinicians’ vigilance. As illustrated by our data, this clearly adds more activity for laboratory involvement in arboviruses diagnosis, since during the surveillance period the number of requests for arbovirus assays more than doubled (Fig 1).
The lack of specificity for clinical signs associated with arboviral infections adds further complications to the identification of locally acquired cases. Most of the suspected cases were not subsequently associated with any specific diagnosis, and more than half of the cases were labelled as fever of unknown origin as observed in previous studies [Reference De Kleijn, Vandenbroucke and Van Der Meer12]. Our observations also confirm that many infectious aetiological agents share the same clinical features with DENV, CHIKV or ZIKV. These agents include common community-acquired viral diseases such as parvovirus B19 or cytomegalovirus infections, various infectious syndrome (sepsis, meningitis, gastroenteritis) or, as expected, other local vector-borne diseases like Mediterranean Spotted fever (Table 2). This illustrates that many differential diagnoses may interfere with that of arbovirus infections and that the overestimation of suspected autochthonous cases would be very difficult to reduce without impairing the chances of recovering confirmed autochthonous cases. However, a strict respect of the suspected case definition could improve the pertinence of diagnosis since in our studies some criteria were lacking for up to 35 % of the tested patients.
The increasing frequency of requests for arbovirus diagnosis in patients not returning from epidemic/endemic areas results from the presence of a competent vector in our region. Although no autochthonous cases have been detected during 2015 in patients seen at our hospital, the same preparedness plan has allowed the rapid identification and management of a small seven-case DENV outbreak in the neighbouring town of Nîmes [Reference Succo13]. Furthermore, regarding the threat of the recent increasing spread of ZIKV, the same preparedness plan should be reinforced and improved each year.
ACKNOWLEDGEMENTS
We thank Dr Isabelle Leparc-Goffart at the National Reference Centre for Arboviruses (Marseille, France) for help with Zika virus serological investigations.
DECLARATION OF INTEREST
None.








