INTRODUCTION
Ecological reservoir studies are an essential component of any integrated public health response to emerging zoonotic diseases in humans [Reference Mills1]. Puumala virus (PUUV) (genus Hantavirus, family Bunyaviridae) is considered an emerging rodent-borne virus due to its increasing significance as a human pathogen [Reference Vorou, Papavassiliou and Tsiodras2, Reference Klempa3]. Within northern and continental West-Central Europe, this virus is the major cause of a specific form of haemorrhagic fever with renal syndrome (HFRS), called nephropathia epidemica (NE). The incidence of NE is related to the geographical distribution of its sole specific reservoir host, the bank vole (Myodes glareolus) [Reference Vapalahti4]. Soon after bank voles become infected with PUUV, they start shedding infectious virus via their excretions. Even though the voles remain chronically infected, the shedding of virus peaks at about 3 weeks after infection [Reference Gavrilovskaya5, Reference Hardestam6]. PUUV is mainly horizontally transmitted through direct contact or indirectly via virus-contaminated environment. The latter transmission mode is also assumed to mediate occasional transmission of PUUV to dead-end hosts like humans, potentially causing NE. This disease is marked by a sudden onset of fever and headache, followed by gastrointestinal problems, vomiting and abdominal pain often accompanied by visual disturbances. Half of NE cases also show renal symptoms, although acute renal failure is only detected in severe cases. The incubation period can vary from a few days up to 41 days. Due to the varying severity of PUUV infections, only 5–10% of infected humans experience clinical problems severe enough to seek medical help. The number of NE cases is increasing and a record number of cases have been reported during the last five years, e.g. in Finland, Sweden, Germany and Belgium [Reference Vapalahti4, Reference Heyman7].
Incidence of NE shows a clear variation in time and space. Regarding temporal variation, a sudden increase in the bank-vole population is often used to explain NE epidemics [Reference Davis, Calvet and Leirs8, Reference Sauvage9]. This idea is based on the mass action principle of disease transmission, assuming that PUUV transmission is density dependent and a higher number of individuals leads to a higher contact rate in bank voles and in humans [Reference McCallum, Barlow and Hone10]. However, within-year seasonal variation in NE incidence can also depend on vole behaviour (e.g. entering human dwellings) or human behavioural patterns related to increased infection risk (e.g. professions like forestry, farming, the military, harvesting crops or outdoor activities, and the cleaning of cellars, stables or summer cottages) [Reference Olsson11–Reference Winter14]. Therefore, short-term PUUV infection dynamics in the reservoir host and NE incidence do not necessarily coincide (see also [Reference Olsson11]). Empirical data covering actual NE epidemics and related local bank-vole dynamics are rare, but highly valuable as they are crucial in explaining NE disease patterns.
Spatial variation of NE cases is also related to local human behavioural patterns and local environmental differences [Reference Linard15]. Yet, a predominant factor determining the local PUUV infection risk for humans is local incidence of infectious bank voles [Reference Davis, Calvet and Leirs8, Reference Sauvage9]. The spatial distribution of PUUV in bank voles is believed to be highly patchy. Similar to the proposed spatial system of Sin nombre virus in deer mice, areas of preferred bank-vole habitat and suitable microclimate conditions that harbour resilient bank-vole populations with a high intrinsic PUUV transmission rate are hypothesized to function as refugia, or persistently infected areas. It is from these areas that PUUV infection could spread during high-density years [Reference Mills and Childs16–Reference Glass18]. A current challenge is to identify the characteristics that are associated with those PUUV refugia. The significant role of environmental parameters in the determination of local PUUV infection risk in bank voles and humans has recently been shown [Reference Linard19]. Besides environmental factors, local population structural and community parameters can also determine spatial variation in PUUV infection in bank voles. This was also suggested for hantavirus infection in hispid cotton rats in Florida and deer mice in Colorado [Reference Glass20, Reference Calisher21]. In general, bank-vole PUUV prevalence increases with age, reproductive activity and is higher in males than females [Reference Escutenaire17, Reference Bernshtein22–Reference Tersago24]. As a consequence of this, local prevalence and transmission rates can be affected by local age structures, sex ratios and proportion of reproductive voles [Reference Glass20]. Moreover, local bank-vole numbers have been found to relate to the local number of PUUV antibody-positive voles [Reference Olsson25] and abundance thresholds, below which PUUV infection can not be maintained, have been suggested in several studies [Reference Tersago24, Reference Escutenaire26]. At the community level, other resident non-host rodent species can negatively affect local transmission rates and prevalence, e.g. by reducing encounters in bank voles or by reducing the number of susceptible bank voles due to inter-specific competition [Reference Keesing, Holt and Ostfeld27, Reference Dizney and Ruedas28].
The present study focuses on the NE–PUUV–bank-vole system in Belgium, where the highest number of NE cases until now was recorded during 2005 [Reference Heyman29]. Before, during, and after this NE outbreak, nine bank-vole populations were monitored in an area with high NE incidence. First, we describe the 2005 PUUV epizootic and compare the data of PUUV infection in bank voles with the epidemiology of regional and local NE cases during the study period. These comparisons allow us to describe the temporal and spatial relationships between NE incidence and PUUV infection in the reservoir host. Second, we investigated effects of selected bank-vole population features on PUUV antibody prevalence and number of PUUV antibody-positive bank voles throughout the study period. This approach allows us to identify bank-vole population or community features that are related to incidence of high local vole PUUV infection rates.
METHODS
NE data
NE data were provided by the Scientific Institute of Public Health (IPH) [Reference Tersago30]. Detailed data were provided for the period 1996–2006. NE patients were georeferenced according to their residential municipality in the IPH database. Data on the annual human population size for each municipality were provided by the National Institute of Statistics (NIS).
Site selection, sampling and serology
Site selection was based on criteria of local NE incidence and preferred bank-vole habitat. The total incidence (NE cases/100 000 inhabitants from 1996 to 2003) and the frequency of NE cases (absence/presence per year from 1996 to 2003) were calculated for all Belgian municipalities. Based on these calculations, out of the 10 highest ranked municipalities, three ‘high NE risk’ municipalities were selected. Within a radius of 60 km of these high-incidence areas, six other municipalities with lower or no NE incidence pattern were chosen (Fig. 1). Subsequently, within the borders of these nine selected municipalities, independent trapping areas were selected with the help of local forest agents (Fig. 1). This selection was based on criteria of broad-leaved forest patches with dense ground vegetation, i.e. the preferred bank-vole biotope in West-Central Europe [Reference Escutenaire17, Reference Mazurkiewicz31]. Sites were located within a radius of 50 km from core: 4° 43′ E, 50° 16′ N. In each municipality, one 10×10 trapping grid was built with 100 Sherman live traps spaced at 10-m intervals. Rodent trapping started in summer 2004 and was repeated every spring, summer and autumn until autumn 2006 (except summer 2006 due to extremely low abundances) each time during three consecutive trap nights per site. Each season, the nine sites were monitored within a 4-week period, in order to minimize temporal effects. The timing of trapping sessions is indicated in Figure 2. Captured bank voles were anaesthetized with Isoflurane (Forene, Abbott, UK) and gender, sexual condition, body weight (±0·5 g) and body length (±1 mm) were noted. Males with their testicles in scrotal position, pregnant and lactating females, and females with a perforated vagina were considered reproductively active. After blood sample (60 μl) collection from the retro-orbital sinus, the animals were individually marked and released at their original place of capture. Serum was obtained after centrifugation (5 min at 5000 rpm) and stored in liquid nitrogen in the field, and later at −80°C in the laboratory. Collected serum was screened by immunofluorescence assay using spot slides with acetone-fixed Vero E6 cells non-infected and infected with Puumala Sotkamo strain (HaartBio Ltd, Finland) as previously described [Reference Tersago24].

Fig. 1. A representation of nephropathia epidemica (NE) incidence in each municipality where a study site was situated. The NE incidence (=number of new cases/100000 inhabitants per time period) is given for the period from 1996 to 2003 and for the three years of the study period separately. The number of NE cases from 1996 to 2003 in each municipality is indicated above the incidence bars. The three municipalities with the highest NE risk are indicated by an asterisk (*).

Fig. 2. Temporal distribution of nephropathia epidemica (NE) cases at the Belgian national level and the regional level of our study (provinces Hainaut and Namur) in combination with regional (all study sites combined) number of PUUV IgG-positive bank voles/ha, PUUV IgG prevalence and bank-vole numbers/ha in each trapping session with respective standard errors. Significant seasonal differences for each variable are marked by different letters A, B, C, D (P<0·05).
Statistical analysis
We performed Generalized Linear Model (GLM) analyses in SAS® v. 9.2 (SAS Institute Inc., USA) for evaluation of overall temporal differences in seasonal bank-vole PUUV IgG prevalence (logistic model), bank-vole numbers and number of PUUV IgG-positive bank voles (log-linear model corrected for overdispersion). A Generalized Estimation Equation (GEE) method was used to correct for repeated measurements in seasons. Between-season differences were assessed using the least square means statement. The relationship between local NE cases/incidence and seasonal number of PUUV IgG-positive rodents was analysed using Spearman's rank-order correlations in SAS.
GLMs in SAS were also used to test the relationship between bank-vole PUUV IgG prevalence (logistic model)/number of PUUV IgG-positive rodents (log-linear model) and local, site-specific population features within each season. Trapping index, sex ratio, proportion of voles with body weight >15 g (weight distribution), proportion of reproductively active voles and sex ratio of reproductively active voles were used as measures of local vole population structure. Fifteen grams was used a cut-off weight value as this is the lowest weight limit from where distinct higher PUUV antibody prevalence was found in a previous study within the same study region [Reference Escutenaire26]. In the analysis, we used the trapping index:
as a measure of bank-vole abundance as it corrects for Sherman traps that closed without a vole entering [Reference Escutenaire26]. However, bank-vole number/ha and trapping index were highly correlated (Spearman: r=0·998, P<0·0001). The number of PUUV IgG-positive bank voles was calculated, based on bank-vole numbers and PUUV IgG prevalence, as not all dead animals were tested for antibodies. Besides bank voles, wood mice (Apodemus sylvaticus) and yellow-necked mice (Apodemus flavicollis) were frequently found in our traps. We used the estimate of
as a measure of Apodemus sp. proportion per field site. Study sites where ⩽4 bank voles per session were captured, were excluded from all proportion analyses and low bank-vole numbers in 2006 compelled us to exclude these data from most of the analyses.
RESULTS
Bank-vole numbers and PUUV infection patterns
Clear changes were observed in the local bank-vole populations and PUUV infection features during the study period (Fig. 2). From summer 2004 to autumn 2004 a significant increase in PUUV IgG prevalence (χ12=48·64, P=<0·0001) and number of PUUV IgG-positive bank voles (χ12=7·23, P=0·007) was found, this change was followed by an additional increase in prevalence in spring 2005 (χ12=8·78, P=0·003), resulting in a higher number of PUUV IgG-positive bank voles (χ12=10·44, P=0·001). Bank-vole abundance did not change significantly in these seasons (P=0·287). In summer 2005, only bank-vole abundance increased significantly (χ12=9·33, P=0·002). Next, a significant decrease in PUUV IgG prevalence (χ12=6·46, P=0·011) and abundance (χ12=7·37, P=0·007) and, hence, also in the number of PUUV IgG-positive bank voles (χ12=11·78, P=<0·001) was recorded in autumn 2005, followed by a sharp fall in bank-vole numbers (χ12=27·84, P⩽0·0001) in spring 2006. During the latter trapping period, despite equal trapping efforts, no bank voles were trapped in four field sites, the highest trapping number recorded was 11 bank voles/ha and only one PUUV IgG-positive bank vole was found. In autumn 2006 numbers had increased again slightly (χ12=6·69, P=0·010) and eight PUUV IgG-positive bank voles were found in two field sites (Fig. 3).
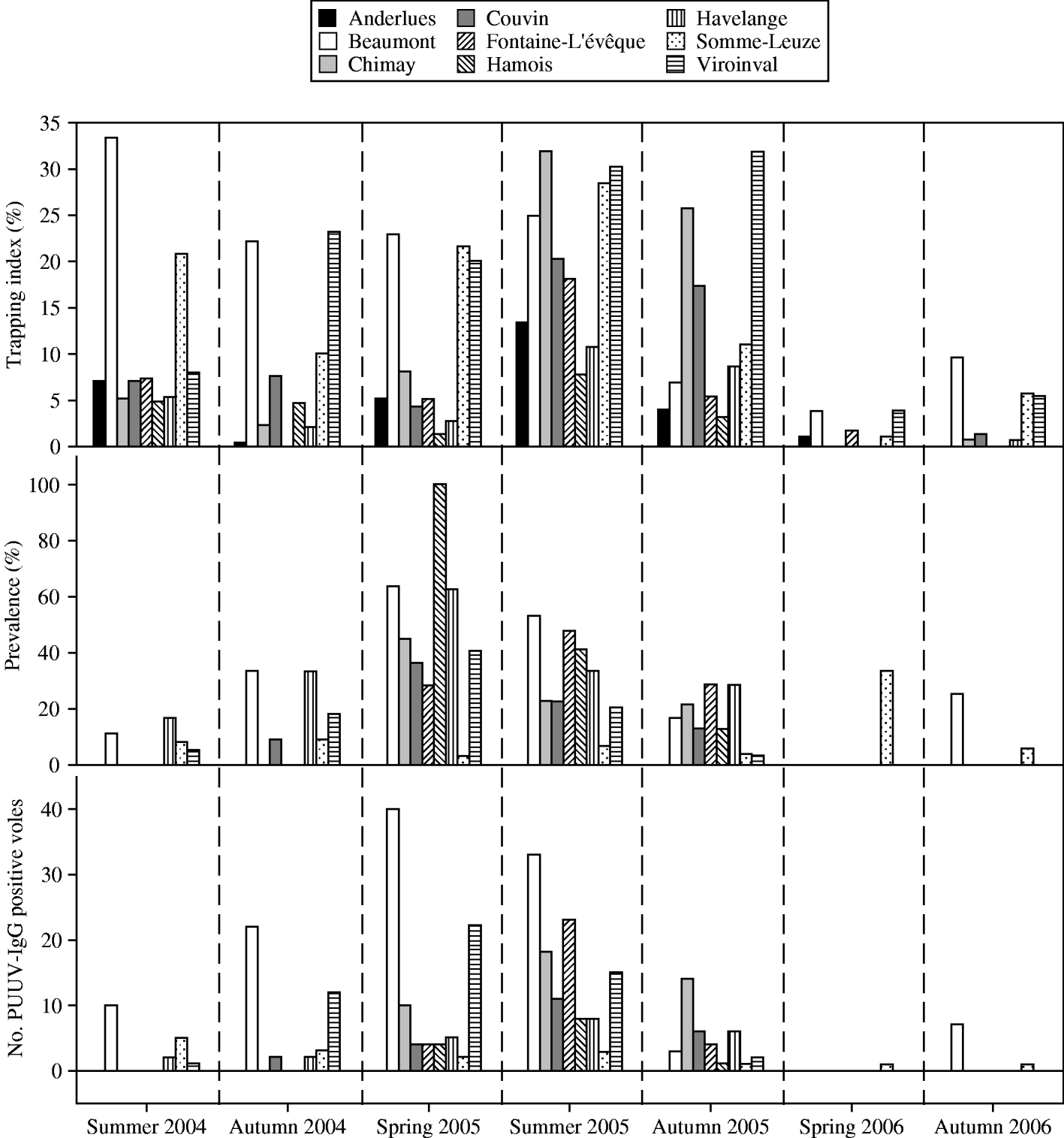
Fig. 3. Results of bank-vole trapping in nine sites in southern Belgium from summer 2004 to autumn 2006. The figure represents seasonal local trapping index, PUUV antibody prevalence (number of bank voles tested) and the number of PUUV antibody-positive bank voles per site (1 ha).
The spatial pattern in the detection of PUUV infection coincided with the above seasonal patterns. In summer 2004, PUUV IgG-positive bank voles were found in 4/9 sites, this increased to five sites in autumn 2004, while a maximum of eight sites with PUUV infection was reached in spring 2005. This distribution was still maintained in autumn 2005 until bank-vole populations fell during winter 2005/2006 (Figs 2, 3).
Variation in NE cases, 2004–2006
Throughout the year, incidence of NE cases in Belgium was previously described as showing a bimodal peak with a minor winter peak between January and March and a major summer peak between June and September [Reference Heyman32]. This pattern remained during our study period, although a small additional autumn peak was present in all three years and in 2006 both winter and summer peaks were equal in size (Fig. 2).
Local NE incidence related to local reservoir host PUUV infection
Figure 1 shows the historical NE incidence (1996–2003) and annual incidence during the study period for each municipality where a field site was situated. We found that in 2004 no PUUV IgG-positive voles were found in sites situated in municipalities with the highest NE incidence rates in Belgium. In contrast, the highest number of PUUV IgG-positive bank voles in 2004 was found in Beaumont, yet no human NE cases occurred locally in this municipality at that time. Significant correlations were only found between the local annual number of NE cases in 2005 and the number of PUUV IgG-positive bank voles in summer 2005 (r=0·69, P=0·039), autumn 2005 (r=0·801, P=0·009) and between the number of NE cases in 2006 and the number of PUUV IgG-positive bank voles in summer 2005 (r=0·721, P=0·028) and autumn 2005 (r=0·797, P=0·010).
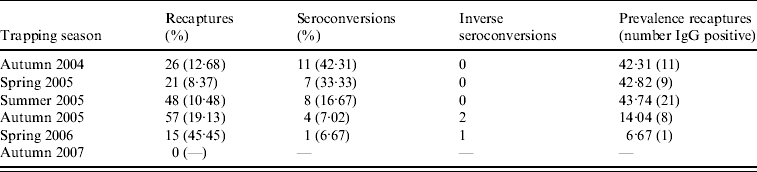
Recaptures, seroconversions and abundance thresholds
Throughout the study period, some patterns in recaptures and bank-vole seroconversions were found, which provide a measure of PUUV transmission frequency in voles (Table 1). In spring 2006, only 33 bank voles were trapped in all nine study sites and of these, 15 were also caught in the previous autumn. This percentage of bank-vole recaptures between sessions (45·5%) was the highest throughout our study. Only one of these recaptured bank voles was PUUV IgG-positive in autumn 2005 and this one juvenile animal (body weight 10 g) had probably lost its maternal antibodies in spring 2006 (body weight 16 g). The highest percentages of seroconversions in recaptured voles were found from autumn 2004 to spring 2005 (33·3%) and from summer 2004 to autumn 2004 (42·31% concentrated in only five field sites) (Table 1, Fig. 3). During the PUUV infection peak in 2005 no indication of an abundance threshold was recorded. PUUV infection was found in the bank-vole populations independent from local bank-vole abundance, and high infection prevalence (100%) was found in a site where only four bank voles were caught (Fig. 3). However, in 2004 except for one field site (Havelange), PUUV IgG-positive bank voles were found in the four field sites containing the highest number of bank voles (Fig. 3).
Table 1. Recaptured bank voles in each season with respective seroconversions to PUUV IgG positivity, inverse seroconversion (disappearance of maternal antibodies) and PUUV IgG prevalence within the recaptured bank-vole population
Local bank-vole population features in relation to PUUV infection
In each season, site-specific characteristics were analysed in relation to PUUV antibody prevalence and absolute number of PUUV IgG-positive rodents (Table 2). A positive relationship between trapping index and number of PUUV IgG-positive voles was found in summer 2004, autumn 2004 and spring 2005. In the period leading towards the 2005 outbreak, the number of PUUV IgG-positive voles was thus related to vole abundance. Only in summer 2004 was a positive relationship between prevalence and trapping index detected. In autumn 2004 the proportion of reproductively active male bank voles appeared to positively influence the number of PUUV IgG-positive bank voles and PUUV prevalence. However, similar relationships were not found in the later trapping seasons. The relative proportion of Apodemus sp. did not affect antibody prevalence or the number of antibody-positive bank voles, independent from the number of bank voles captured in a site (overall P>0·05).
Table 2. Parameter estimates of site-specific population features (trapping index) and proportion of reproductively active (RA) males that relate significantly to the number of PUUV IgG-positive bank voles (log scale) and PUUV IgG prevalence (logit scale) within one season

N represents the number of study sites used in the analysis.
DISCUSSION
Our results show that the peak of NE cases in our study region occurred when the highest number of PUUV IgG-positive rodents was present in the populations. However, due to the chronic nature of PUUV infection in voles, presence of PUUV IgG antibodies does not necessarily reflect recent PUUV infection [Reference Vapalahti4]. Analyses of the recaptured bank voles showed that most seroconversions occurred during the increasing phase in 2004 and from autumn 2004 to spring 2005. Hence, our data suggest that the highest transmission rate in voles precedes the peak in NE cases. The incubation preceding NE symptoms and human outdoor activity patterns during the summer months probably influenced the NE incidence pattern [Reference Vapalahti4]. This relationship differs from the situation in northern Europe, where the highest peak of NE cases appears in late autumn and winter, probably due to bank voles entering human dwellings owing to harsh winter conditions [Reference Olsson11, Reference Olsson33, Reference Kallio34]. However, an inconsistency was found in 2006 when a small NE peak was recorded early in the year, even though extremely low numbers of bank-voles were present and almost no PUUV infection was detected. A tendency of lower than average winter temperatures was recorded during the beginning of 2006 and these winter conditions remained until the end of March 2006 (Climate report 2006, Royal Meteorological Institute, Belgium). These conditions may have led to specific human or vole behaviour that increased infection risk, e.g. voles seeking shelter in human dwellings, and more use of firewood stacks stored outside, where old, virus-contaminated vole excretions are in optimal conditions for remaining infectious [Reference Olsson11, Reference Kallio35].
On the local spatial level, our data only represent one focus within a municipality. We found that high local numbers of PUUV IgG-positive voles do not necessarily lead to local NE infection (see Beaumont, summer 2004). If this pattern is consistent throughout other high NE incidence areas, changes in land use can easily result in increased infection risk for humans, even during non-epizootic years. For example, new human suburbs that encroach forests and where new gardens are situated close to high PUUV infection patches are likely to result in high PUUV infection risk for the new resident human population. This local pattern is similar to what we found in northern Belgium, where PUUV infection occurs in local bank voles, but NE cases are rare. However, in this northern region an overall lower PUUV antibody prevalence (1·79% in 2004 and 3·54% in 2005) was recorded and the 2005 epizootic was less pronounced [Reference Linard15, Reference Tersago24]. By contrast, in the southern high-NE incidence study region, we found a sharp increase in PUUV prevalence and spread of PUUV infection throughout almost all study sites from autumn 2004 to spring and summer 2005. During this PUUV epizootic, local numbers of PUUV IgG-positive voles in summer 2005 and autumn 2005 were positively correlated with the annual number of NE cases in each municipality in both 2005 and 2006.
Our observations support the hypothesis that during hantavirus outbreaks, infection spreads from refuge patches to neighbouring forest patches, thereby causing an overall regional distribution of PUUV infection [Reference Glass18, Reference Escutenaire26]. We aimed to identify general vole population structural features that identify patches with incidence of high PUUV infection. We found that the proportion of sexually active males during autumn 2004 is positively associated with the local number of PUUV IgG-positive bank voles and PUUV prevalence. Further, sex ratio was positively correlated to incidence of PUUV infection in a monitoring study in the northern region of Belgium [Reference Tersago24]. These findings are in line with our expectations as adult males have been suggested to be important hantavirus carriers and transmitters due to their greater mobility, aggressiveness (rate of direct contacts) and their apparent ability to shed more hantavirus [Reference Escutenaire17, Reference Klein36, Reference Klein and Calisher37]. Our results do not confirm the existence of a dilution effect on PUUV prevalence due to the relative proportion of non-host Apodemus sp. However, such a dilution effect was described in the low relative risk area for NE in northern Belgium [Reference Tersago24]. Increased importance of the indirect transmission route in the high-NE risk area could be a possible explanation for the absence of this effect in our study sites. Different soil types and climatic conditions in this study region have been hypothesized to allow for longer survival of the virus outside the host, hence minimizing potential effects of encounter reduction due to other non-host species [Reference Linard19, Reference Keesing, Holt and Ostfeld27, Reference Kallio35].
The number of PUUV IgG-positive rodents was positively correlated with the local bank-vole trapping index until spring 2005. Before the epizootic, in summer 2004, prevalence was also increased in patches with high trapping index. Hence, within an area of high NE incidence, a large number of local bank voles is an important feature of those sites with high PUUV incidence or a high intrinsic transmission rate. The fact that no relationships were found in the subsequent seasons, suggests that, above a certain level of infection, local population features (i.e. local bank-vole abundance and sex ratio) no longer played a major role in determining local PUUV infection risk.
Within the range of densities that we observed, our data do not suggest the existence of a vole abundance threshold necessary to allow invasion of PUUV. Yet, we do find indications for the existence of a threshold for persistence of PUUV infection [Reference Davis, Calvet and Leirs8, Reference Lloyd-Smith38, Reference Swinton, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson39]. Vole populations fell over winter 2005/2006 and PUUV-infected voles remained virtually absent in the following spring (Fig. 3). In contrast, we found a high recapture rate in spring 2006 of only uninfected voles captured in the previous autumn. Decreased winter survival in bank voles due to PUUV infection was demonstrated in a previous study and might have influenced the observed sharp fall in bank-vole numbers [Reference Kallio40]. In autumn 2006, again eight PUUV-infected voles were detected, but only in those two sites that showed the highest bank-vole numbers. Interestingly, these two sites also showed the highest number of PUUV IgG-positive voles before the epizootic in summer 2004. Long-term studies during multiple non-epizootic years are necessary to identify the actual persistence of this infection status.
In a previous study we showed the importance of high tree-seed production, triggered by warm-weather conditions, for the prediction of high numbers of NE cases in our study region [Reference Tersago30]. In the present study, we now find that the exceptional peak in NE incidence during 2005 was directly associated with increased incidence of PUUV infection in bank voles on both the spatial and temporal level. As expected, observed high tree-seed production in autumn 2004 created good conditions for bank voles which led to increased numbers of bank voles in the following spring and favoured PUUV transmission. Our findings suggest that, after such high tree-seed production in autumn, PUUV infection spreads readily from potential PUUV refugia to neighbouring patches, causing high spatial incidence of PUUV infection at the beginning of the breeding season in spring. We found that in the beginning of such an epizootic, local high bank-vole numbers can be used as an indication of PUUV high-risk patches. These results suggest that a well targeted artificial reduction of local bank-vole numbers during autumns with high tree-seed production could be used as a preventive measure against PUUV spreading in high-NE risk areas.
ACKNOWLEDGEMENTS
We especially thank local forest agents and the Walloon Department of Nature and Forests for trapping permission and support in the field. We also appreciate the help of the many field assistants. We are grateful for the scientific support of the UK's Joint Environment and Human Health Programme (funded by NERC, Defra, EA, MOD and the MRC). This research was partially funded by EU grant GOCE-2003-010284 EDEN and is catalogued by the EDEN Steering Committee as EDEN0115 (www.eden-fp6project.net).
DECLARATION OF INTEREST
None.









