INTRODUCTION
Measles is a disease fully preventable through different vaccination policies such as the universal two-dose strategy, the catch-up of susceptible subjects or by extemporal programmes of mass vaccination. Even so, measles is still endemic in the European region and the measles virus (MV) continues to spread due to poor controls. Thus, the goal of eliminating measles in Europe by 2010 proved to be unattainable [Reference Lopalco and Martin1]. The main reason for this failure is the less than optimal vaccine coverage in several European countries. Although in the past decades considerable efforts were made and spectacular results attained, the current measles epidemiology is of concern to public health. In the period 2006–2009 EUVAC-NET reported the resurgence of widespread epidemics in both Eastern Europe (Rumania, Bulgaria) and Western Europe (UK, Switzerland, Austria, Germany, France) [Reference Muscat and Bang2–Reference Muscat4].
In Italy, universal vaccination against measles, mumps and rubella (MMR) has been recommended since the early 1990s for all children at age 15 months, with a varying degree of compliance nationwide. In addition, in 2003, the National Plan for Measles and Congenital Rubella Control launched by the Italian Ministry of Health introduced two other strategies, i.e. the universal second dose at 5–6 years and a catch-up of children attending primary school [Reference Bonanni5–Reference Spiteri, Fenech Magrin and Muscat8]. Despite these efforts and the considerable decrease of morbidity since 1995, measles is still endemic in Italy and since 2006 a number of outbreaks have occurred in a number of regions, namely Apulia, Lazio and Piedmont; these outbreaks were caused by two distinct genotypes, D4 and B3 [Reference Caputi9–Reference Curtale13].
This survey reports on an epidemic that occurred in the farthest region of northeast Italy (Friuli Venezia Giulia, FVG), describing the epidemiological pattern, assessing the molecular features of the responsible virus and mapping its transmission pathway.
MATERIALS AND METHODS
Data collection and case definition
In Italy, measles is a mandatory notifiable disease. In 2008, any notified case with the typical clinical presentation was registered as a suspected case at the Prevention Department of the Health Districts ASS 6 and ASS 1. The administrative boundaries roughly correspond to the provinces of Pordenone and Trieste, respectively, with a total of about 500 000 inhabitants. Suspected cases were interviewed over the telephone or asked to complete a questionnaire on demographic data and history of exanthemata, vaccinations and contacts. Following informed consent, patients provided clinical samples for laboratory testing.
A confirmed case was defined as a patient with documented seroconversion, with or without virus detection/isolation in organic fluids. A probable case was defined when a clinically suspected case without laboratory evidence had an exposure to a confirmed case. Cases considered as suspected on grounds of sole clinical features were not included in this survey.
Serology
Diagnostic serology was performed with a measles IgG and IgM EIA commercial kit (Dia-Sorin, Italy). Measles infection was established when IgM antibody was unequivocally present. In the event of an equivocal result, a second serum sample was required to ascertain seroconversion.
Virological and molecular evaluation
Virological and/or molecular evaluations were carried out on throat swabs, urine and peripheral blood collected in EDTA. Nucleic acids were extracted with NucliSens easyMag System (bioMerieux Italia, Italy) and measles RNA detection was performed with a nested RT–PCR. The sequences of the 520-bp amplicon in the carboxy-terminal variable region of N gene (Nucleoprotein nt 1252–1722) was obtained by a hemi-nested PCR using a specific sequencing primers set [Reference Chibo14].
Pharyngeal swabs and urine were seeded on B95 cell culture, incubated for 10 days and submitted to one blind passage. Supernatants of cell cultures with or without cytopathic effects were tested with RT–PCR.
For sequencing, the amplicons were concentrated and desalted using the Microcon 100 device (Amicon, USA), and were sequenced by Big Dye Terminator chemistry, v. 3.1, using sequencing primers under standard conditions [Reference Chibo14]. Reaction products were analysed by ABI 310 Genetic Analyser (Applied Biosystems, USA). The assemblage of sequences was performed by the Sequencer package 4·5 of Gene Codes Corporation (USA). Phylogenetic and molecular analyses were performed using MEGA version 4.0 [Reference Tamura15]. The phylogenetic tree was constructed by the neighbour-joining method on amino-acid sequences; the Kimura two-parameter method was used to calculate nucleotide substitutions, and a bootstrap of 500 replicates confirmed the significance of the tree's topology. A search for highly similar sequences was performed with the Megablast Algorithm [Reference Zhang16]. Measles reference strains [17–19] and previously reported sequences of measles N protein from GenBank were included in the phylogenetic tree. Sequences generated in this study were deposited in GenBank under the following accession numbers: HQ687077–HQ687086.
Statistics
Data are presented as frequency, proportion or mean, as appropriate. The χ2 and Fisher's exact tests were used to test differences in frequencies and Student's t test to assess differences in mean. The differences were considered significant when P<0·05.
RESULTS
From 7 April to 25 May 2008 46 measles cases, defined according to the established criteria, were recorded by the Regional Public Health Department of FVG Region. Forty-two cases were distributed between two contemporary but distinct outbreaks occurring in the Pordenone area (ASS 6 Friuli Occidentale) and in the city of Trieste (ASS 1), 110 km away (see Fig. 1).

Fig. 1. Measles cases in Friuli Venezia Giulia region by day of onset of symptoms and administrative area (ASS 1, Trieste area; ASS 6, Pordenone area).
The outbreak in Trieste comprised 33 cases. Primary cases (four unvaccinated teenagers) occurred among students attending an instruction tour to the Piedmont region (31 March–3 April 2008) where a measles outbreak was in progress. After returning home, one boy in the last incubation day attended a christening along with some families in which parents and children were unvaccinated. As a result, an infection cluster of 14 secondary cases occurred both among the members of those families and their contacts.
The ASS 6 Friuli Occidentale outbreak involved nine cases. The index case was a previously unvaccinated 32-year-old teacher who probably acquired the infection during a tour of Austria and Germany. In particular, she returned home on 24 March after a 4-day visit to Munich and felt ill on 31 March. Eight secondary cases mainly involving family members were then reported.
Morbidity data are shown in Table 1. The mean age of the cases was 17 years (median age 15 years, range 0–62 years). The most affected age cohort was 15–19 years, with an incidence rate of 25/100 000. Male:female ratio was about 1:1 (22 female, 20 male). Of the 42 cases, 40 (95·2%) were unvaccinated, most of them belonging to families where the parents had refused vaccinations. Two patients were hospitalized with pneumonia and one case of otitis was reported.
Table 1. Measles morbidity by age and gender

Table 2 shows the vaccine coverage in this region just before the epidemic onset distributed by health districts. The overall proportion of vaccinated children was 93·4% for the first dose and 88·3% for the second dose. It should be noted that the lowest coverage was recorded in the area of Trieste.
Table 2. MMR vaccine coverage by administrative area in the Friuli Venezia Giulia region, NE Italy

Bold indicates areas involved in the outbreaks.
* Cohort of 2005.
† Cohort of 2001.
Clinical specimens (urine, throat swab, serum) for laboratory confirmation were obtained from 15 cases. In two cases, measles-specific IgM antibodies were detected in serum, without virus identification. The measles virus was found in 13/15 cases by RT–PCR. Cultures led to the isolation of seven strains which were submitted for sequencing, while another three strains were sequenced directly. All nucleoprotein sequences were similar and the virus was classified as genotype D4. The phylogenetic tree of the 10 sequences is presented in Figure 2 together with some reference and recent sequences available in Genbank. The epidemic strains showed a strong relationship to strains isolated in Genoa and Florence during the 2008–2009 epidemic and were believed to be acquired in Piedmont, as well as to the strain circulating in the UK, namely the D4 MVs/Enfield.GBR/14.07. No corresponding sequence from Germany was retrieved from Genbank.
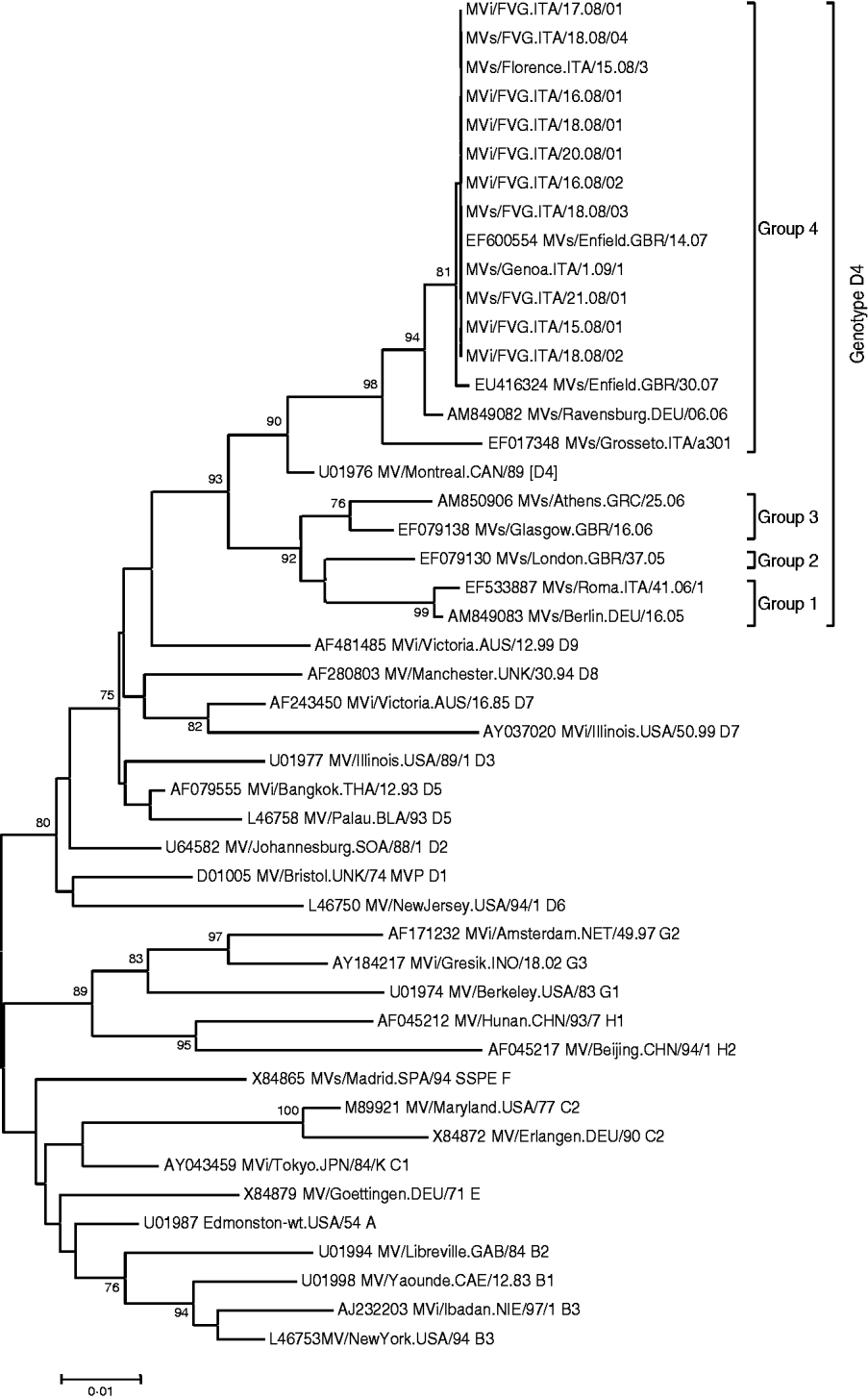
Fig. 2. Phylogenetic tree of the sequences obtained in the study compared to reference viruses [19] and sequences obtained from GenBank. The phylogenetic tree was calculated on the basis of the 456 nucleotides that code for the C-terminus of the N protein, by using MEGA 4 ·0 software and the neighbour-joining method (500 bootstraps). Reference strains and sequences from GenBank are indicated with the accession number followed by the strain name according to World Health Organization nomenclature.
DISCUSSION
After the large Italian epidemics of 2002 and 2003 that affected 62 and 29 cases, respectively, only space-temporal scattered cases of indigenous measles have been identified by the regional network for epidemiological and laboratory surveillance. An unusual genotype, namely the D5 genotype, was imported from Thailand in 2005 and generated only one secondary case in a family contact. Thus, in this area, the morbidity rate was very low, <0·1 case/105 inhabitants [Reference Muscat and Bang2, 20], until the present outbreak occurred.
The epidemiological analysis indicated the independent introduction of two measles viruses spreading in other epidemic areas, i.e. Piedmont [Reference Filia10, Reference Filia11] and Germany [Reference Bernard, Fischer and Wild21, Reference Schmid22]. In both outbreaks the index case was linked to educational settings where the virus affected a small number of susceptible subjects. All interested schools were public and no anthroposophic theory was currently offered in these educational settings [Reference Schmid23, Reference van Velzen24]. An additional spread of measles virus occurred during a religious ceremony involving some families in which the parents had refused vaccinations for themselves and their children due to ideological reasons [Reference Cohuet25].
The outbreaks affected males and females at an equal rate, while the most affected age group was aged 15–19 years. It is of note that about 20% of infections occurred in adults (aged >25 years). Hence, the resulting age-shift was more marked than that recorded in the global epidemiology of the European region, where young children belonging to marginal or difficult-to-reach populations were frequently infected [Reference Muscat4].
Collectively, the epidemiological profile described here could be due to the level of MMR vaccine coverage. Children in the second year of life are homogenously vaccinated at a proportion of 93%, quite close to the targeted figure of 95%, and the MMR second dose was delivered to 88% of children, a higher rate than that recorded in other Italian areas where large epidemics occurred. This finding could explain the containment and the decline of the epidemics, particularly in the Pordenone area.
Genotyping of isolated measles viruses showed that the two epidemics were sustained by genotype D4. This genotype has a vast geographic distribution, including the Indian subcontinent, South/East Africa, and the Middle East [Reference Kremer26]. Genotype D4 strains circulating in Europe were able to be distinguished into four groups with a NT variability ranging between 5% and 2% [Reference Kremer26]. The strains detected and those epidemiologically related fitted group 4 (Fig. 2). The strict homology with the genotype circulating in Piedmont and imported from the UK (i.e. MVs/Enfield.GBR/14.07) was confirmed, hence providing the molecular evidence of epidemiological features [Reference Ansaldi27]. In contrast, clear epidemiological and molecular evidence of the second outbreak onset did not emerge. The first index case became ill with measles exactly 7 days after returning from a tour of central Europe that concluded in Bavaria. Since a strain indistinguishable from the UK D4 genotype continued to sustain a large outbreak at the end of 2007 in Bavaria, it seems plausible that the virus acquisition could have occurred there [Reference Bernard, Fischer and Wild21, Reference Schmid22]. This hypothesis is supported by the continued detection of genotype D4 during the first months of 2008 in some German areas (Dr Santibanez, personal communication). Unfortunately, the epidemiological and molecular proof for this hypothesis could not be established with certainty.
In conclusion, the goal of eliminating measles seems attainable in this region where a high rate of vaccination coverage has already been attained. To avoid recurrence of outbreaks, a continuous effort should be made to achieve the optimal vaccine coverage of children and to offer MMR vaccine to difficult-to-reach populations. In addition, a robust laboratory assessment should support any measles surveillance programme [Reference Muscat28]. Laboratory evaluation is needed to confirm clinical diagnosis in a very low endemic period when the positive predictive value of the clinical features is poor. In addition, molecular evaluation becomes essential in tracing epidemiological pathways and distinguishing between imported and autochthonous strains.
ACKNOWLEDGEMENTS
We thank Dr Filippo Ansaldi of the Department of Health Sciences, University of Genoa for the sequences of Genoa and Florence strains and Dr Sabine Santibanez of the WHO Regional Reference Laboratory for Measles and Rubella at the Robert Koch Institut, Berlin, for information on genotypes circulating in Germany in 2008. We thank Mrs Claudia Biagi, Fabia Petronio and Elena Samar for their excellent technical assistance.
DECLARATION OF INTEREST
None.








