INTRODUCTION
Congenital cytomegalovirus (CMV) infection is the most frequent intrauterine infection and the main non-genetic cause of sensorineural hearing loss in infancy [Reference Fowler and Boppana1]. The incidence of congenital CMV infection and disease vary according to the epidemiological and immunological characteristics of the population of women of childbearing age [Reference Kenneson and Cannon2]. High rates of congenital CMV infections have been consistently found in populations with a high seroprevalence [Reference Kenneson and Cannon2]. Anticipating the risk of CMV vertical transmission and planning preventive measures are facilitated by knowing these characteristics.
Most of the available CMV seroprevalence data for Brazilian pregnant women are a decade old and were derived from hospital-based studies [Reference Suassuna, Leite and Villela3, Reference Pannuti4]. Although a large study based on neonatal universal screening showed a high prevalence of congenital CMV infection in infants born to low-income women from the southeast region of Brazil [Reference Mussi-Pinhata5], the maternal age-specific CMV seroprevalence rate in this population is not yet known.
The main objective of this study was to determine the age-specific prevalence of serum antibodies for CMV in a representative sample of unselected pregnant women from a population utilizing the public health system in Ribeirão Preto, a city located in the southeast region of Brazil in the state of São Paulo.
METHODS
The study population consisted of pregnant women belonging to the low- to middle-income stratum who received prenatal care from the unified public health system (SUS). Sample size estimates were based on published CMV seroprevalence data [Reference Almeida6] using 95% confidence intervals (CIs) with a desired 2% precision. These parameters yielded a minimum sample of 1063 pregnant women. The sample was stratified into the following age groups: 12–19, 20–24, 25–29, and ⩾30 years. From September 2005 to September 2006, a total of 4615 pregnant women were assisted in the 36 primary-care public units in Ribeirão Preto. The number of pregnant women to be enrolled per age group in each urban residential district was determined on the basis of the proportion of pregnant women observed in each district (East, West, South, North, Centre).
Serum samples from pregnant women were obtained from a general repository in a public health laboratory consisting of anonymous residual serum specimens, which were obtained during prenatal care after routine serological tests were performed. The specific samples to be tested from each district and age group were selected via a computer-generated random number list. The first sample obtained during pregnancy from each participant was selected. Birth data, the date the sample was taken, and information about local residence were collected from each woman. No data concerning parity, number of living children, or professional or marital status were available.
The study was approved by the Research Ethics Committee of the University Hospital (Process no. 1952/2009). A waiver of informed consent was obtained from this Committee.
Laboratory assays
A commercial enzyme immunosorbent (Vidas; bioMérieux, France) was used for the quantitation of specific IgG CMV antibodies. Sera samples with antibody titres <4 AU/ml were considered negative; titres from 4 AU/ml to <6 AU/ml were considered equivocal; and titres ⩾6 AU/ml were considered positive. The results were quantitatively expressed according to the manufacturer's instructions. Sera samples with titres in the equivocal range were retested once, and the sera samples that were still in the equivocal range were presented as equivocal.
Data analysis
The data obtained were analysed by the SAS system, version 9.2 (SAS Institute Inc., USA). Seroprevalence was determined according to the age groups of interest with 95% CI calculations. Testing for associations between CMV antibody seropositivity and age group was performed using Fisher's exact test. Arithmetic means and mean deviations were calculated for the CMV antibody titres from each age group. An analysis of variance (ANOVA) was performed after the natural logarithmic transformation of CMV antibody titres according to age group was conducted.
RESULTS
The median age of the pregnant women was 24 years (range 12–46 years). Serum samples for 75/1063 (7%) participants were not available and could not be tested. A total of 988/1063 (92·9%) participants had available samples for the quantification of CMV IgG antibodies. Of the 988 pregnant women, 29 (2·9%) were seronegative and three (0·3%) had antibody titres in the equivocal range. Thus, the overall estimate of CMV seroprevalence in 985 pregnant women was 97% (95% CI 95·8–98·0). The proportion of seropositive pregnant women in each age group is shown in Table 1. There was no significant increase of CMV seropositivity based on age. Additionally, the CMV seroprevalence rates did not differ significantly between women from the five different residential districts; the rates ranged from 95·3% in the North to 96·8% in the Centre district.
Table 1. Age-specific CMV seroprevalence in 985 pregnant women
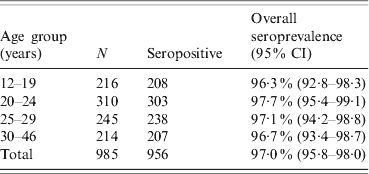
CI, Confidence interval.
Fisher's exact test (P value = 0·78).
The mean values (standard deviations) of CMV antibody titres (AU/ml) for the 12–19, 20–24, 25–29, and ⩾30 years age groups were 82·3 (39·4), 79·5 (44·7), 83·7 (49·5), and 90·6 (44·5), respectively. Figure 1 shows the distribution of these titres according to age group. Overall, the antibody titres varied according to age group (P = 0·04). However, only the titres detected in the ⩾30 years age group were found to be significantly higher than those in the 20–25 years age group (mean difference –0·15, 95% CI –0·24 to –0·05, P < 0·01).

Fig. 1. Boxplots of CMV antibody titres (AU/ml) after the natural logarithmical transformation according to age group.
DISCUSSION
Adding to the knowledge of CMV epidemiology in Brazilian pregnant women from a population with a high-birth prevalence of congenital CMV infection [Reference Mussi-Pinhata5], we found that CMV seroprevalence is almost universal (97%) and similar in pregnant women from four age groups ranging from 12 to 46 years, even in those in the youngest age group (12–19 years). These findings are consistent with those detected in pregnant women in other developing countries [Reference Tabatabaee and Tayyebi7–Reference Cannon, Schmid and Hyde9] and suggest that the majority of primary CMV infections occurs early in this population, i.e. in infancy or childhood. By contrast to rates found in developed countries, where ∼50% of pregnant women are CMV seropositive [Reference Tookey, Ades and Peckham10–Reference Dollard12], our results indicate that ∼95% of pregnant women became naturally seroimmune by a median age of 18 years. These findings are in accordance with our previous findings of the high birth prevalence of congenital CMV infection in this population, as it has been consistently demonstrated that congenital CMV infection rates increase with maternal seroprevalence due to transmission from maternal recurrent infections [Reference Wang13, Reference Yamamoto14]. Furthermore, although this study is restricted to pregnant women, the detected age prevalence profile is consistent with that observed in Brazilian blood donors [Reference Souza15] and suggests that most women from this population are no longer susceptible to primary infection with this virus during childbearing age.
The early occurrence of primary CMV infection in this population can probably be explained by conditions predisposing to frequent CMV exposure in childhood and adolescence. A retrospective survey for CMV antibodies in an urban Brazilian community showed that the first increase in CMV seropositivity was observed before age 5 years, when 60% of individuals had detectable CMV antibodies. At age 15 years there was a second increase in this seropositivity, suggesting a sexual component for the transmission of the virus [Reference Almeida6]. The high percentage of seropositive mothers is expected to be the main source of infection for young infants either through breastfeeding, which is practised by 85% of mothers by age 4 months and 55% by age 1 year [Reference Pereira16], or by means of close contact with mother's secretions such as saliva and urine. We have previously detected CMV viruria in 38·2% of non-congenitally infected infants from this population aged 3–6 months [Reference Yamamoto, Figueiredo and Mussi-Pinhata17]. After the second year of life, non-maternal sources of infection may enhance viral transmission. Close contact between children at home or at day-care centres, and a high number of people in the household may be possible modes of viral spread during childhood. In addition, nearly half of Brazilian adolescents have had their first sexual intercourse at a mean age of 15 years, irrespective of gender [Reference Borges, Latorre and Schor18].
With respect to viral antibody titres, the overall relative stability of CMV antibody levels between the younger and older age groups detected in this study is a likely consequence of the ability of CMV to cause recurrent infections and the frequent viral exposure within this population. Whereas antibody titres elicited after infection with viruses such as rubella, for which long-lasting protective immunity is produced, tend to decrease over time [Reference Aksakal19], we found a tendency towards higher levels of CMV antibody in the youngest age group with a slight decrease in women from the 20–24 years age group and an increase from this point in the older age groups. However, the difference of titres was only statistically demonstrated between women aged 20–24 years and those aged 30–46 years. These features could be explained by more recent primary infections in the youngest women and the boosting of the immune response following viral reactivation [Reference Mehta20], or natural exposure to multiple CMV strains in older women (who therefore may have had more intense and prolonged antibody response to the cumulative viral reactivation or exposure over time) [Reference Stowe21]. In view of these findings, and considering our previous demonstration that congenital CMV disease also occurs in this population [Reference Mussi-Pinhata5, Reference Yamamoto22] at a similar frequency to its occurrence in populations in which only half of the women of childbearing age are susceptible to primary infection [Reference Staras23–Reference Bate, Dollard and Cannon25], it is likely that boosting the antibody production using vaccines against CMV will not be enough to confer protection against congenital CMV infection and/or disease in highly seroimmune populations. Thus, a better understanding of the virus–host interaction, viral immune evasion mechanisms during gestation, and the factors that facilitate maternal–fetal transmission of this virus in women from these populations are still warranted.
In conclusion, we demonstrated that most low-income pregnant women utilizing the public health system of a southeast Brazilian city are CMV seropositive from an early age. Nationally representative CMV seroprevalence and epidemiological studies, which should additionally include women from higher-income backgrounds, are needed to evaluate the risk factors for primary and non-primary infections in women of childbearing age and pregnant women. These studies will be crucial before a specific strategy for the prevention of congenital CMV infection and/or disease, such as immunizing with vaccines currently under development [Reference Sung and Schleiss26], can be made available.
ACKNOWLEDGEMENTS
This work was supported by the Fundação de Apoio à Pesquisa, Ensino e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA), Brazil. We are grateful to Cláudia Siqueira Vassimon, supervisor of the Central Health District Castelo Branco, Ribeirão Preto, for her assistance in providing the prenatal serum specimens stored in the central repository.
DECLARATION OF INTEREST
None.






