Different studies have analysed the prevalence of hepatitis C virus (HCV) infection in people with HIV, with a high variability in the seroprevalence reported. Rates of HCV are consistently higher in areas and regions where the HIV epidemic is sustained by injecting drug use (IDU), and markedly lower in countries where IDU represents an uncommon route of HIV transmission [Reference Santiago-Munoz1–Reference Bao and Liu7]. In Africa, where most of HIV transmission is attributable to unprotected sexual intercourse, seroprevalence rates of HCV are relatively low, usually below 5% [Reference Madhava, Burgess and Drucker8, Reference Otegbayo9]. Even if the recent evolution of HIV epidemics has been characterized by a growing number of people of African origin in several European countries [Reference Hamers10, Reference Prost11], there is very limited information on the possible impact of this phenomenon on HCV seroprevalence rates, and on recent trends in HCV prevalence in pregnant women with HIV in these countries. The issue is relevant, because HCV progression is usually accelerated by HIV co-infection [Reference Thein12], and pregnant women with HIV-HCV co-infection have a significantly higher risk of transmitting HCV to their offspring compared to women with HCV infection only [Reference Tovo13–Reference Polis15]. A reduction in HCV seroprevalence in women with HIV is therefore expected to decrease the number of paediatric cases of HCV infection. Given the importance of this issue and the limited information available on trends in HCV-HIV co-infection in pregnant women, we decided to examine recent temporal trends in HCV seroprevalence in pregnant women with HIV, assessing the contribution of women of African origin to these trends.
We used data from the National Programme on Surveillance on Antiretroviral Treatment in Pregnancy, an ongoing national observational study on pregnant women with HIV established in Italy in 2001 to collect information on the safety and efficacy of antiretroviral treatment in pregnancy [Reference Floridia16]. Informed consent is required for all enrolled women, using a patient information sheet that has received approval by the competent Ethics Committee. Based on anonymous seroprevalence studies [Reference Girardi17], it is estimated that the study collects about half of all deliveries from HIV-infected mothers occurring in the country. For the current analysis, data were extracted from the general database on 15 May 2009, and included all pregnancies reported between 2001 and 2008 with available information on HCV status, determined by HCV antibody testing with commercial enzyme-linked immunosorbent assays. HCV RNA data were not collected.
Quantitative data were compared by Student's t test for independent samples; categorical data were compared using χ2 test, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated according to Mantel–Haenszel estimates. Trends over time were assessed by χ2 test for trend, and the association between HCV and the variables found to be significant in univariate analyses were assessed using a multivariable logistic regression model, which calculated adjusted odds ratios (aORs) and 95% CIs for HCV infection (dependent variable), using maternal age, route of transmission, geographical origin, months from HIV diagnosis and calendar year as model covariates. All P values <0·05 were considered statistically significant. The analyses were performed using SPSS software, version 17.0 (SPSS Inc., USA).
A total of 1240 pregnancies reported between 2001 and 2008 had available data on HCV serology. Overall, 302 women were HCV positive, for a seroprevalence rate across the entire period of 24·4%. Of 1226 women with available provenance data, 306 (25%) were of African origin, and of women of non-African origin, 771 (83·8%) were from Italy, and 149 (16·2%) from other countries (Europe: 91/149, 61%). The overall proportion of women with history of IDU was 18·4%. Non-African origin, HCV infection, and past IDU were all significantly associated with an older age (32·8 vs. 29·9 years for non-African vs. African origin; 34·0 vs. 31·4 years for HCV positive vs. HCV negative, and 34·6 vs. 31·2 years for IDU vs. no IDU; all P values <0·001) and with a longer time from first diagnosis of HIV infection (82·0 vs. 25·1 months for non-African vs. African origin; 112·5 vs. 53·8 months for HCV positive vs. HCV negative, and 128·7 vs. 58·8 months for IDU vs. no IDU; all P values <0·001).
HCV seroprevalence showed a significant decrease during the study period, from 29·3% in 2001 to 8·6% in 2008 (P<0·001). This trend was accompanied by a parallel decline in the proportion of women reporting IDU, from 25·8% in 2001 to 3·0% in 2008 (P<0·001), and by an opposite trend for the proportion of women of African origin, which increased from 21·2% in 2001 to 48·6% in 2008 (P<0·001, Fig. 1 a). HCV co-infection was very uncommon in African women (1% vs. 32·0%, OR 0·021, 95% CI 0·007–0·066, P<0·001), and no women in this group reported IDU as a possible route of infection.
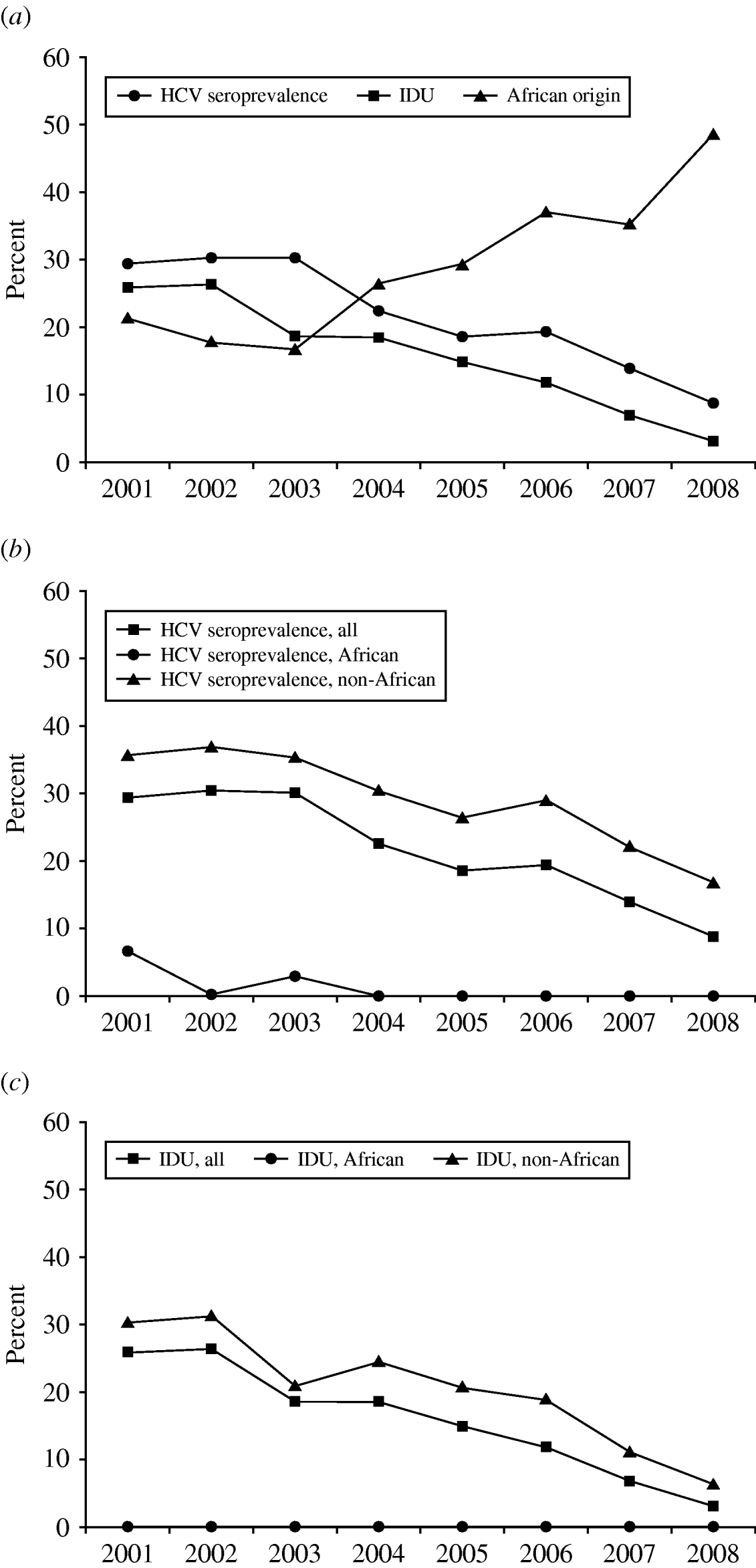
Fig. 1. (a) HCV seroprevalence, history of injecting drug use (IDU), and African origin in the population studied: trends 2001–2008. (b) HCV seroprevalence by geographical origin, 2001–2008. (c) History of IDU by geographical origin, 2001–2008.
Prevalence rates of HCV infection during the study period (2001–2008) showed distinct trends according to the origin of the women. Of the 306 women of African origin, a stable low prevalence of HCV was observed (average seroprevalence rate 1%), with only three HCV-positive cases observed between 2001 and 2008 (two in 2001, one in 2003). Of the women of non-African origin, HCV co-infection was much more common, and seroprevalence rates decreased significantly during the study period, from 35·7% in 2001 to 16·7% in 2008 (P=0·003, Fig. 1 b). Prevalence and temporal trends for IDU were also completely different between women of African and non-African origin: throughout the study period no women of African origin reported history of IDU (prevalence 0%), while the prevalence of past IDU declined significantly in non-African women, from 30·4% in 2001 to 6·3% in 2008 (P<0·001, Fig. 1 c).
In univariate analyses, African origin was inversely associated with HCV co-infection (OR 0·021, 95% CI 0·007–0·066, P<0·001), while IDU was strongly associated with HCV co-infection (OR 54·5, 95% CI 33·9–87·5, P<0·001).
The results of the multivariable analysis confirmed, after adjusting for cofactors, that the risk of HCV co-infection was significantly lower for women of African origin (aOR 0·09, 95% CI 0·03–0·29, P<0·001) and significantly higher for women with a history of IDU (aOR 30·9, 95% CI 18·8–51·1, P<0·001) or a longer interval from diagnosis of HIV infection [aOR for unitary change (months) 1·005, 95% CI 1·002–1·009, P=0·002]. No significant effects were observed for age (P=0·768) or for calendar year (P=0·381).
A few studies have explored the issue of HCV prevalence in pregnant women with HIV [Reference Santiago-Munoz1, Reference Landes5, Reference Jamieson6]. However, none of the above studies have explored recent trends in HCV seroprevalence in this particular population, or the effect introduced by mixing of different populations.
We have described a situation which can be considered relatively common in several European countries, where women of African origin represent an increasing proportion of pregnant women with HIV. We showed a progressive decline in the prevalence of HCV co-infection in pregnant women with HIV, confirming in this particular population the same decline recently described in the general population of people with HIV in another European country [Reference Pérez Cachafeiro18].
We have provided further information on this issue, quantifying the reduction in HCV seroprevalence in pregnant women with HIV over the last few years, which corresponds to a 3·4-fold reduction between 2001 (29·3%) and 2008 (8·6%). We also showed that this decline can be considered the net result of two components: a progressively declining HCV seroprevalence in non-African women (from 35·7% in 2001 to 16·7% in 2008), sustained by a parallel reduction in the proportion of IDU in this population, and a significantly growing proportion of women from a population with a minimal HCV seroprevalence contributing to the total number of pregnant women with HIV. We also quantified in multivariable analysis adjusted for cofactors the lower risk for women of African origin (aOR 0·09) and the higher risk for women with a history of IDU (aOR 30·9), also showing that the risk of being HCV co-infected increases with the time from diagnosis of HIV.
These results have different implications. IDU remains the main factor associated with HCV co-infection, and screening and prevention programmes aimed at reducing the incidence of new HCV infections, in the setting of both horizontal and vertical transmission, should therefore be reinforced. Nonetheless, the observed trend in HCV prevalence is encouraging, because it indicates a declining seroprevalence not only as a consequence of the introduction of a low-prevalence group within the entire population of pregnant women with HIV, but also as an independent effect in the subgroup of non-African women, where seroprevalence halved between 2001 and 2008. Even if we did not assess HCV RNA levels, which are an important marker of HCV transmission to the newborn [Reference Thomas19], these changes overall suggest that a reduced number of cases of vertical HCV transmission is to be expected. Unfortunately, this is an issue that we could not assess, because of incomplete data on HCV testing in newborns, and that should be evaluated in large prospective studies of children from HIV-infected mothers. Another important issue is represented by the definition of trends in HCV seroprevalence in non-HIV-infected pregnant women, in order to clarify whether the decline in HCV seroprevalence is limited to the group of HIV co-infected women or is a general phenomenon involving the entire population of pregnant women with HCV.
APPENDIX
Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy
Project coordinators: M. Floridia, M. Ravizza, E. Tamburrini.
Participants: M. Ravizza, E. Tamburrini, P. Ortolani, F. Mori, C. Monticelli, E. R. dalle Nogare, G. Sterrantino, M. Meli, S. Polemi, J. Nocentini, M. Baldini, G. Montorzi, M. Mazzetti, B. Borchi, F. Vichi, E. Pinter, E. Anzalone, R. Marocco, V. S. Mercurio, A. Carocci, E. Grilli, A. Maccabruni, B. Mariani, A. Moretti, G. Natalini, G. Guaraldi, K. Luzi, G. Nardini, A. Zoncada, A. Degli Antoni, A. Molinari, P. Rogasi, M. P. Crisalli, A. Donisi, M. Piepoli, V. Cerri, E. Chiesa, A. Lupo, D. Repetto, A. Viganò, V. Giacomet, V. Fabiano, S. Stucchi, C. Cerini, G. Placido, M. Dalessandro, A. Vivarelli, P. Castelli, F. Savalli, V. Portelli, S. Alberico, C. Businelli, A. Meloni, D. Gariel, C. Cuboni, F. Ortu, P. Piano, A. Citernesi, I. Vicini, E. Periti, A. Spinillo, M. Roccio, A. Vimercati, B. Guerra, F. Cervi, E. Tridapalli, G. Brighi, M. Stella, G. Faldella, C. Puccetti, M. Sansone, P. Martinelli, C. Tibaldi, L. Trentini, S. Marini, G. Masuelli, L. Di Lenardo, I. Cetin, M. L. Muggiasca, V. Conserva, T. Brambilla, E. Ferrazzi, C. Giaquinto, M. Fiscon, R. Rinaldi, E. Rubino, A. Bucceri, R. Matrone, G. Scaravelli, G. Anzidei, S. Di Giambenedetto, C. Fundarò, O. Genovese, C. Cafforio, C. Pinnetti, G. Liuzzi, V. Tozzi, P. Massetti, M. Anceschi, A. M. Casadei, F. Montella, A. F. Cavaliere, V. Finelli, C. Riva, L. Lazier, M. Cellini, S. Garetto, G. Castelli Gattinara, A. M. Marconi, M. Ierardi, S. Foina, B. Salerio, S. Dalzero, M. Moneta, C. Polizzi, A. Mattei, M. F. Pirillo, R. Amici, C. M. Galluzzo, S. Donnini, S. Baroncelli, M. Floridia. Pharmacokinetics: M. Regazzi, P. Villani, M. Cusato.
Advisory Board: A. Cerioli, M. De Martino, P. Mastroiacovo, M. Moroni, F. Parazzini, E. Tamburrini, S. Vella.
SIGO-HIV Group National Coordinators: E. Ferrazzi, P. Martinelli.
ACKNOWLEDGEMENTS
This work was supported by public grants 39C/A, 31D55, 31D56 from the Italian National Programme on Research on AIDS. We thank Cosimo Polizzi and Alessandra Mattei for providing technical secretarial for this study, Carmela Pinnetti for her valuable help in data retrieval, and all the women who participated in this study.
DECLARATION OF INTEREST
None.





