INTRODUCTION
Shigellosis is usually transmitted from person to person in households, although outbreaks due to contaminated food or water are not uncommon [Reference Mandell, Bennett and Dolin1–3]. Mediterranean countries are likely to be an endemic area of shigellosis. Waterborne outbreaks related to this microorganism have been reported from countries like Israel, Spain and Greece [Reference Alamanos, Maipa, Levidiotou and Gessouli4–Reference Morera, Espejo and Coll8]. Shigellosis appears to be a problem for both developed and developing countries, although the majority of Shigella-related morbidity occurs in the developing world [3, Reference Ford9].
Outbreaks of shigellosis that occur in Mediterranean countries may not be reported as they occur in communities that are distant from water-control centres, or in countries where surveillance systems are not well developed. As a consequence, we do not know the real dimensions of the problem, even in Mediterranean countries with relatively satisfactory environmental hygiene measures and control of drinking or recreational water.
The study of the epidemiological characteristics and environmental conditions associated with outbreaks caused by known waterborne pathogens is important, as this knowledge may be helpful for investigating other outbreaks due to uncertain causes, as well as for the prevention and control of those outbreaks. Since aetiological agents are not identified in many outbreaks, even in countries presenting a high level of surveillance, identification of a waterborne source is frequently based on epidemiological evidence [3, Reference Graun, Nwachuku, Calderon and Craun10].
In addition, environmental, climatological, and socioeconomic factors may be associated with observed attack rates, the shape of epidemic curves, the distribution of cases among the groups of the population exposed, and the duration of the outbreak.
In Greece, the public health surveillance for most infectious diseases, including shigellosis, is not well developed at the national level. Surveillance is based on a system of obligatory declaration of identified cases by the physician making the diagnosis to the regional public health authorities. This surveillance system is characterized by an important underestimation of the occurrence of many infectious diseases. A small number of shigellosis cases are reported every year in the whole country. However, it seems that Shigella was responsible for a considerable number of sporadic cases, and for a number of outbreaks occurring in relatively large communities and even in small cities, that remain unidentified [Reference Alamanos, Maipa, Levidiotou and Gessouli4, Reference Samonis, Elting, Skoulika, Maraki and Tselentis7].
In this article we present a comparative study of four community waterborne outbreaks of shigellosis which occurred in different places in Greece during the last three decades. These are the only outbreaks of waterborne shigellosis that were reported in Greece during this period. The aim of this study is to examine epidemiological and environmental characteristics of the outbreaks so as to evaluate the likely cause of their occurrence. This can be an important tool for public health and environmental protection by preventing the occurrence of similar outbreaks.
MATERIALS AND METHODS
In this study we present and compare all community waterborne Shigella outbreaks that were identified in Greece, after 1970.
Public health surveillance of infectious diseases in Greece is based on a system of obligatory declaration of identified cases. The physician making the diagnosis of shigellosis has to report the case to the regional public health authorities within 24 h. It is estimated that a large number of shigellosis cases are not recorded through this surveillance systems. Only a few tens of cases are reported annually over Greece [11].
During the last decades a number of waterborne outbreaks that occurred in several places throughout the country have been studied by University Departments, which investigated these outbreaks independently from public health authorities. The results of these studies have been published in scientific journals or Congress Proceedings.
To identify all outbreaks of shigellosis reported in Greece after 1970, we searched the Medline database, medical Congress records, Greek medical journals and laboratory records from the University Laboratories of Hygiene. Four community waterborne outbreaks of gastroenteritis attributed to Shigella were identified and studied in the period after 1970 [Reference Alamanos, Maipa, Levidiotou and Gessouli4, Reference Samonis, Elting, Skoulika, Maraki and Tselentis7, Reference Vlachos, Stathopoulos, Katsougiannopoulos and Edipidis12, Reference Katsougiannopoulos, Maipa, Kouniakis and Kagalou13].
The fields compared concerned the geographical region, socio-demographic characteristics of the affected areas, environmental, seasonal and climatological conditions, attack rates, epidemic curves, age and sex distribution of gastroenteritis cases, hospitalization rates, and laboratory studies.
Cases
This study was based on a systematic review of published results. There was access to investigation records that could allow for more detailed evaluations only for outbreaks II and IV.
The sources and methods used to ascertain and analyse outbreaks were different for each study. For outbreaks II and IV, the institution that performed the study was the Laboratory of Hygiene, Medical School, University of Ioannina, which provided immediate access to investigation records, whereas in outbreaks I and III, the institutions that performed the studies were the Laboratory of Hygiene, University of Thessaloniki and the Medical School of Heraklion, Crete respectively. From these we used evidence from written reports.
In outbreak I, a random sample of all cases identified by local doctors was analysed. In outbreak II, all cases identified by local doctors or referred to a local hospital were included in the study. In outbreak III, a list of suspicious cases was compiled from information supplied by the local doctor, the mayor, relatives and neighbours of the patients. These suspicious cases were evaluated by the study group, in order to establish the final list of cases recorded. In outbreak IV, the sources used to identify cases were in- and outpatients referred to the two local hospitals. The final list of cases was confirmed by the study group. In all studies an epidemiological report was completed including information on personal characteristics, residence, clinical characteristics of the disease and day of disease onset. Several other issues were also recorded which differed among the studies.
Environmental studies
Environmental conditions related to the outbreaks were obtained from published reports [Reference Alamanos, Maipa, Levidiotou and Gessouli4, Reference Samonis, Elting, Skoulika, Maraki and Tselentis7, Reference Vlachos, Stathopoulos, Katsougiannopoulos and Edipidis12, Reference Katsougiannopoulos, Maipa, Kouniakis and Kagalou13]. The responsible authorities for conducting the environmental studies were the local public health authorities, but in all cases the University Departments conducted an independent environmental study.
The water supply system and the sewage network were inspected in all cases. A relatively detailed description of the water sources, tanks and distribution systems was available for every outbreak. Demographic data of the four communities at the time of the outbreak were presented by the study groups. The study groups also presented data concerning the climatological conditions, general hygiene measures taken to control the water supply systems, and measures taken during the first days after the appearance of the outbreak.
Laboratory studies
In order to identify an aetiological agent, sampling was performed in stool, and water specimens. Several ways of microbial identification were used such as serological and biochemical methods of identification, bacteriological and plasmid DNA examination (outbreak III), API 20E profiling (outbreak IV), and antimicrobial susceptibility test (outbreaks III and IV) [14, Reference Sambrook, Fritch, Maniatis and Nolan15].
RESULTS
The main characteristics of the four outbreaks are shown in the Table.
Table. Characteristics of the four outbreaks
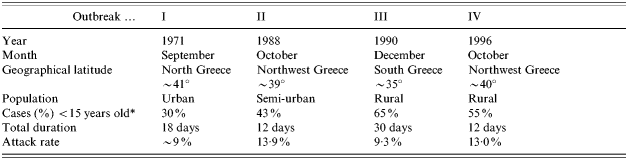
* For outbreak III, the percentage listed is for cases <12 years of age.
Laboratory studies
Shigella sonnei was isolated in both stool and water samples in outbreaks I, II, and IV. In outbreak IV the same plasmid- or antibiotic-resistance profile of S. sonnei was identified in all specimens examined. S. sonnei isolated from water samples had the same resistance profile. In outbreak III S. sonnei was isolated from stool specimens, and the plasmid profile showed three different plasmid patterns, presenting the same antimicrobial susceptibility profile.
Environmental conditions
Two of the outbreaks (I, II) occurred in towns, with populations of approximately 27 000 and 7000 inhabitants respectively at the time of the outbreak. These towns were district capitals. The two other outbreaks (III, IV) took place in largely rural communities of more than 1000 inhabitants. Outbreak IV occurred in a community located nearby the capital of the district. Outbreaks II and IV occurred in neighbouring areas of the country (Northwestern Greece), whereas outbreak I occurred in Northern Greece and outbreak III in Southern Greece.
There was a seasonal variation in their appearance going from September (in the North of the country) to December (in the South). Outbreaks II and IV (which took place in neighbouring areas of Northwestern Greece) occurred at the same seasonal period and almost at the same dates, although almost a decade apart.
There was a strong suspicion of contamination of the water distribution system from the sewage network, in all four outbreaks. In outbreak I the water distribution system and the sewage network were not in a satisfactory condition. The water supply was obtained from two fountains. One of them was located in the town and at lower level than neighbouring residences. The water collections were uncovered and exposed to any kind of environmental pollution. Inappropriate chlorination was also mentioned.
In outbreak II, heavy rainfall was reported a few days before the outbreak, at the same time that work was being performed on the water distribution system. Thus, a possible contamination of the network system from sewage or ground surface material was suspected.
In outbreak III, the affected village was located on a hill with valleys on each side. The water and sewage systems were in relatively good condition. The water supply for the village was obtained from three fountains, two located on a hill higher than the village and a third located in a valley at a lower elevation. The water from the three fountains was collected by pumps in a central tank, located on a hill higher than the village and then distributed to the houses. Unprocessed sewage material was coming out at a distance of 600 m from the valley fountain.
In outbreak IV, the community water system served almost the whole population of the village affected, using ground water from a single borehole as one source. The system was not regularly chlorinated and controlled. The sewage network was in a satisfactory condition. The well was situated near a milk factory and some residences outside the village. Heavy rainfall was reported a few days before the outbreak.
All outbreaks were controlled almost immediately after proper environmental control measures had been taken.
Epidemiological features
The epidemic curves of the four outbreaks are presented in the Figure. Based on environmental conditions and the distribution of cases, the four outbreaks seemed to correspond to two different ‘models’ of epidemic spread, which are likely to be related to specific environmental conditions.

Fig. Epidemic curves. 1st Model: outbreaks II, IV (attack rates ∼13%). 2nd Model: outbreaks I, III (attack rates ∼9%).
In the first model, the outbreak began suddenly, presenting a peak at the first or second day followed by 2 days with high attack rates. Three days after the peak, the curve began to decline significantly. Some late and sporadic cases appeared from day 10 to day 20, probably due to person-to-person spread (outbreaks IV and II). Heavy rains preceded the outbreaks. The attack rates were relatively high, ∼13% for both outbreaks.
In the second model, there was an initial small peak of the epidemic at the first or second day. After this few cases were recorded for some days. An escalation in the number of cases was observed, reaching a second and higher peak after a few days. Then, the number of new cases declined quickly. Most cases occurred 1–3 weeks after the start of the outbreak. No heavy rainfall was noted. These outbreaks had lower attack rates, ∼9% in both cases, and water intakes were located below the towns.
Data collected in outbreaks II, III, and VI show similar attack rates for both sexes. There are no data available on the sex distribution for outbreak I. Children <15 years of age comprised the largest proportion of cases in all outbreaks, ranging from 30% of cases in an urban setting to 65% of cases in a rural setting (Table).
Hospitalization rates, which represent the severity of the disease, were reported only in outbreaks IV and II, and were greater among children and the elderly. The clinical expression of shigellosis in all four outbreaks included diarrhoeal disease, abdominal cramps, fever, and vomiting. Bloody diarrohea was present in a small percentage of cases (<10%).
DISCUSSION
There are many potential sources of outbreaks of gastroenteritis related to Shigella spp. Several transmission routes can be involved. In some cases the outbreak can be related to more than one transmission route, or to more than one microorganism [16, Reference Maurer and Sturchler17]. The type and the characteristics of an epidemic are related not only to the aetiological agent, but also to the source and the transmission route of the agent, as well as to environmental and socioeconomic factors. Waterborne outbreaks attributed to Shigella continue to be reported in several Mediterranean countries. Their epidemiological characteristics are not well known and there is a lack of studies aiming to give a global epidemiological ‘profile’ of those outbreaks, as well as to investigate the influence of environmental and socioeconomic factors in their expression [3, Reference Graun, Nwachuku, Calderon and Craun10, Reference Cano Portero, Mangas Gallardo and Velasco Munoz18, Reference Lee, Levy, Craun, Beach and Calderon19].
The four outbreaks reviewed were identified as waterborne. They presented some common characteristics, as well as some important differences, concerning their epidemiological features in relation to the environmental conditions associated with the occurrence of the outbreaks. All were caused by S. sonnei. In most cases there was a strong suggestion that contamination of the water system occurred through sewage or surface water run-off.
The outbreaks seem to follow two different models, presenting characteristic epidemic curves related to specific environmental conditions. The two outbreaks in neighbouring areas of Northwestern Greece followed the same patterns as far as the shape of the epidemic curves and seasonal occurrence. The attack rates and the age distribution of cases were similar also. The number of affected individuals began to decline sharply ∼2 days after simple environmental and hygiene measures had been taken. Heavy rains preceded the outbreaks, in the beginning of the rainfall season. It could be assumed that these two outbreaks were related to sudden, but probably not continuous sewage contamination of the groundwater resulting from the heavy rainfall. It is interesting that similar patterns of epidemiological curves were observed in waterborne outbreaks that occurred in other countries [3, Reference Maurer and Sturchler17, 20].
The two other outbreaks seemed to correspond to a second outbreak ‘model’. There was an initial peak of the epidemic at the first or second day, followed by small number cases for some further days. Then an escalation in the number of cases was observed, reaching a second and higher peak after a few days. Most cases occurred 1–3 weeks after the start of the outbreak. No heavy rainfall was noted before the outbreaks. These outbreaks had lower attack rates, ∼9% in both cases, and water intakes were located below the towns. Thus, the implication is for initial, probably low-level contamination of the water system that was probably amplified as more people became ill and contamination levels increased.
The risk of developing gastroenteritis decreased significantly with age and this was an important common point for all epidemics studied. Children and young adolescents presented the highest attack rates (5–10 times higher than adults). Higher incidence of shigellosis among children has been reported in other studies and could be explained by a lower degree of natural immunity to shigellosis, as well as to frequent contacts among children. A higher degree of natural immunity among adults and the elderly could be related to previous exposure to S. sonnei probably associated with prolonged negligence in the control of water systems [Reference Wilson, Feldman, Davis and La Venture21, Reference Cohen, Green, Block, Rouach and Ofek22]. This is also suggested by lower percentages of cases occurring among adults in the rural outbreaks.
Different groups of investigators, using different methods of recording cases, investigated the four outbreaks studied. It is possible that a number of patients, especially patients with mild symptoms were not recorded. They probably represent a different percentage of gastroenteritis cases for each outbreak. Moreover, S. sonnei gastroenteritis is often asymptomatic. Inadequate specification of diagnosis, and different diagnosis criteria might also be a source of bias. Primary and secondary cases were not identified in many cases. It should also be pointed out that it was impossible to compare similarities and differences among strains of S. sonnei responsible for each outbreak in the frame of this study. Those differences could also be related to different epidemiological characteristics among outbreaks. Finally, the study was based on a systematic review of published results, and there was no access to investigation records for all outbreaks. These methodological problems pose some limitations to the interpretation of data, especially data concerning the epidemic curves of the outbreaks. However, there is strong indication that epidemic waves corresponded to two characteristic types of epidemic curves, which are related to specific environmental conditions influencing the occurrence and expression of the outbreaks.
In this regard, it is of interest that all four outbreaks occurred during the month of the first considerable rainfall when sewage or other infected material could be carried by rainwater towards the fountains, or the water distribution system. In addition, all four outbreaks occurred in large villages or towns that mostly represented a rural and semi-urban population, with common water supply systems that were irregularly controlled and chlorinated. The fact that the two more recent outbreaks recorded took place in smaller communities, suggests an improvement in the control of common water systems in larger towns and district capitals in Greece. This highlights the need for sanitary sewage disposal and adequate water treatment and distribution systems for all population centres.






