INTRODUCTION
Since the mid-1990s, methicillin-resistant Staphylococcus aureus (MRSA) infections have been increasingly encountered in community settings worldwide [Reference Herold1–Reference Chambers3]. Multiple dominant clonal lineages have driven this pandemic [Reference David and Daum4]. Despite such rapid dissemination, it remains unclear how community-associated (CA)-MRSA clones spread and become established within communities. Multiple studies conducted across different settings have identified the household as an important reservoir for S. aureus [Reference Wagenvoort5–Reference Uhlemann10]. After a household member becomes infected, high levels of S. aureus colonization and infection often occur in other household members [Reference Zafar11–Reference Fritz15]. Reports have observed that epidemic clones tend to ‘ping-pong’ in family members, resulting in a high rate of recurrent infections [Reference Jones16–Reference Cook18]. Eradicating S. aureus from the household and reducing the frequency of these infections has proven difficult [Reference Fritz19, Reference Miller20]. A greater understanding of how S. aureus spreads in household members is essential for the design of evidence-based prevention and treatment strategies.
Various studies have examined the spread of S. aureus in households in discrete geographical locations [Reference Knox9, Reference Mollema21–Reference Nerby24]. These studies have identified various risk factors associated with household transmission in these distinct settings. However, these studies have been limited to analyses of the S. aureus strains that were predominant in those discrete locations. To date, no study has pooled primary data across multiple countries in order to assess the spread of S. aureus in the household setting. Such an analysis would allow for an examination of heterogeneity in the spread of S. aureus in households and identify common risk factors for household transmission across settings and diverse strain types.
In order to assess these issues, we pooled primary data from three studies conducted in New York, USA (US), Breda, The Netherlands (NL), and Melbourne, Australia (AU) [Reference Knox9, Reference Uhlemann10, Reference van Rijen25]. These studies utilized similar procedures to assess risk factors for household transmission of CA-MRSA in the households of infected cases.
METHODS
Populations
The current study is a retrospective, observational study that pooled primary data from three cross-sectional studies assessing household transmission of S. aureus [Reference Knox9, Reference Uhlemann10, Reference van Rijen25]. These studies used similar methods but were conducted across diverse geographical regions, demographically different populations, and featured unique clinical S. aureus strains. The study locations had similar levels of economic development and population access to healthcare. Table 1 provides a comparison of the characteristics of the three studies. One of the studies (US) sampled exclusively from a major metropolitan area, another study (AU) sampled from a major metropolitan area and the surrounding suburbs, and the third study (NL) sampled from 16 hospitals located throughout the country. In all three studies, a patient with CA-MRSA infection was identified through inpatient and outpatient screening at a hospital (US and NL), or through a community-based private pathology service (AU). In all studies, relevant exclusion criteria were applied to isolate community-associated infections from healthcare-associated infections. Once potential index cases were identified, they were contacted and home visits were scheduled with those who were willing to participate. At the time of the home visit, all household members were asked to participate and provided informed consent. On average, home visits were conducted 61 days (s.d. = 59) after the infection was cultured.
Table 1. Characteristics of included studies in pooled analysis

MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
Procedures
The three studies followed similar procedures. At the time of the home visit, all household members who were willing to participate provided swabs from the anterior nares and completed a questionnaire. Anterior nares cultures were collected with sterile swabs from all consenting household members, excluding children aged <1 year because of the logistical difficulties of swabbing them. Culture swabs were incubated overnight in high-salt 6·5% broth and plated onto selective media agar for 18–48 h at 35–37°C. S. aureus was confirmed by coagulase, Protein A detection kit or both. Methicillin resistance was determined by selective media agar, disc diffusion antibiotic sensitivity testing, or polymerase chain reaction (PCR) was used to test for the presence of staphylococcal chromosomal cassette (SCC)mec. S. aureus-positive isolates were genotyped by spa sequencing [Reference Knox9, Reference Uhlemann10, Reference van Rijen25–Reference Shopsin27]. The clinical infection isolates were also retrieved for all index cases. These isolates were obtained from identified sites of infection and underwent the same analyses as all other isolates.
The questionnaires administered in the three studies captured information on a number of risk factors for CA-MRSA acquisition and household transmission. These variables included sociodemographic information (e.g. age, gender, education, income), index patient community exposures (e.g. work, school, day care, sports participation, travel), health information (recent skin infection, hospital admission, antibiotic use, insulin use), and household characteristics [presence of a pet (dog/cat), presence of children aged <18 years, towel sharing, razor sharing]. Variables shared across all three studies were included in statistical analyses.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All studies were approved by their respective ethical review boards (US: the Institutional Review Board of Columbia University Medical Center; NL: the medical ethics committee of the St Elisabeth Hospital in Tilburg; AU: the University of Melbourne Human Research Ethics Committee).
Measures
Only risk factors assessed in all studies were included in these analyses. Risk factors were categorized as index patient sociodemographic characteristics (e.g. gender, age, born in country, education), index patient acquisition risk factors (e.g. day-care attendance, school attendance, sports participation, international travel), index patient transmission factors (recent skin condition, recent abscess, being colonized with the clinical isolate at the time of the home visit), other household member acquisition risk factors (recent surgery), household transmission risk factors [e.g. presence of a pet (dog/cat), sharing towels, sharing razors], and household sociodemographic characteristics (e.g. household size, percent of children in the household). Acquisition risk factors were considered potential factors that could lead to S. aureus acquisition in the community while transmission risk factors were considered potential factors that could lead to the spread of S. aureus in members of a shared household. In the US study, risk factors were assessed over the previous 6 months. In the NL and AU studies, risk factors were assessed over the previous year. Household transmission was defined as colonization of a non-index household member with the same strain spa type as the index patient's clinical isolate [Reference Miller28].
Statistical analyses
For comparisons of frequencies of index case and household descriptive data by study, χ 2 tests and t tests were used. In analyses comparing households with evidence of transmission to those without on sociodemographic and risk-factor data, Poisson regression models with robust error variance were used to estimate prevalence odds ratios (pORs). pORs are reported instead of traditional odds ratios because of the high prevalence of the outcome in our sample (23%, n = 67) [Reference Knol29–Reference Zou31]. Initially bivariate analyses were run and all variables associated with intra-household S. aureus transmission at P < 0·20 were considered for inclusion in multivariate analyses strategies [Reference Knox9, Reference Miller32–Reference Mickey and Greenland34]. Once these variables were identified, multivariate analyses were used to model transmission in each individual study and effect estimates were compared to look for heterogeneity of effects across studies. Effect estimates were similar across studies. Heterogeneity of effects across studies was also assessed with meta-analyses for each individual risk factor, using the effect estimates and 95% confidence intervals (CIs) to generate a summary effect estimate, as well as a Q statistic, for each risk factor. No heterogeneity was observed (P values for the Q statistics ranged from 0·51 to 0·87) and so the primary data from the three studies were pooled and analysed using a fixed-effects model. Any residual effect of combining data across study sites was controlled for in all models using pooled data through inclusion of study site as a covariate in multivariate analyses. We subsequently repeated these analyses using generalized estimating equations (GEE) analysis in order to account for potential clustering within study site, assuming an independent covariance structure, and observed similar effects to the previous analyses; thus the results of the initial analyses are reported. Additionally, all analyses controlled for household size as a potential covariate. Heterogeneity of effects was also assessed in the pooled data analyses by entering interaction terms for each risk factor by study site in the multivariate model. Again, no heterogeneity of effects was observed and the analyses were run with only main effects. To limit the impact of collinearity, correlations between covariates were examined and it was determined that no variables were correlated enough to affect our models. pORs and 95% CIs are presented. All statistical tests were two-sided and P < 0·05 was considered statistically significant. Data were analysed using SAS v. 9.2 (SAS Institute Inc., USA).
RESULTS
Study population characteristics
The total study sample consisted of 296 index cases and 798 household members. The US study included 139 index cases and 467 household members, the NL study included 61 index cases and 114 household members, and the AU study included 96 index cases and 217 household members. Of the 296 index cases, 44% (n = 131) were male and 24% (n = 70) were aged <18 years. Of those aged ⩾18 years (n = 226), 74% (n = 167) had completed high school. The average household size was 3·7 people (s.d. = 1·7).
Table 2 presents the distribution of index patient- and household-level sociodemographic characteristics, acquisition and transmission risk factors by study. Studies differed on multiple variables. For example, a relatively low proportion of index patients in the US study were born in the US. Moreover, a relatively low proportion of index patients had recent exposure to healthcare settings. In the NL study, a relatively large proportion of index patients and non-index household members had recent exposure to healthcare settings. In the AU study, a relatively large proportion of index patients played sports and had recently travelled internationally. Towel sharing was more common in household members in the AU study and less common in the US study. Index patient colonization with the clinical isolate was more common in the NL study and less common in the US study. In summary, the three studies included very different sample populations with regard to index patient- and household-level sociodemographic characteristics, acquisition and transmission risk factors.
Table 2. Distribution of index patient and household (HH) sociodemographic characteristics and risk factors by study
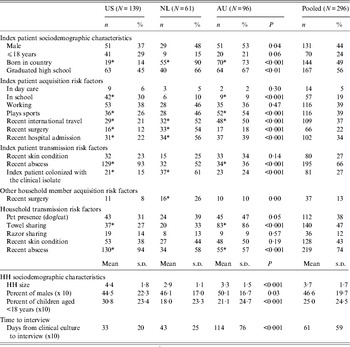
P value refers to test for heterogeneity between studies. χ 2 tests were used for categorical variables and ANOVA for continuous variables.
* Indicates standardized residuals are >1·96 or < − 1·96, thus indicating which specific study is accounting for observed heterogeneity between studies.
Molecular characterization of S. aureus isolates
Overall, there was diversity of index patient clinical strain types between each study. Figure 1 presents the distribution of index patient spa types of clinical isolates by study. In the US study, MRSA t008 (USA300) was the predominant strain type, accounting for 73% (n = 101) of index patient infections. In the NL study, there was a much wider assortment of strain types causing index patient infection, with 29 different strain types accounting for 61 infections and MRSA t008 only accounting for 7% (n = 4) of index patient infections. The most commonly identified strain was MRSA t011, which accounted for 18% (N = 11) of infections. In the AU study, there was not a single predominant epidemic strain. The most common strain types were MRSA t019 (13%, n = 13), MRSA t037 (11%, n = 11), and MRSA t202 (10%, n = 10). MRSA t008 only accounted for 3% (n = 2) of index patient infections. A few strain types were identified across multiple studies, notably MRSA t002 (US: 1%, n = 2; NL: 11%, n = 7; AU: 5%, n = 5), and MRSA t008 (US: 73%, n = 101; NL: 7%, n = 4; AU: 2%, n = 2). MRSA t019 was relatively common in the NL (13%, n = 13) and AU (17%, n = 13) studies.
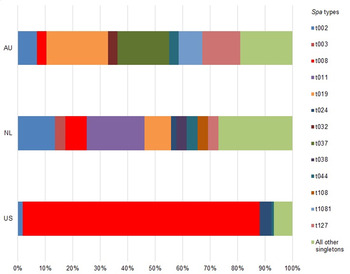
Fig. 1 [colour online]. Distribution of clinical isolate spa types by study.
One fifth (20%, n = 18) of the 97 index patient clinical isolates in the AU study could not be spa-typed because the specimens retrieved from the private pathology service were no longer viable. In these cases, antibiograms run by the private pathology service were used to confirm that the clinical isolates were MRSA. In one of these 18 households, a non-index household member was colonized with MRSA; however, it had a different resistance pattern than the clinical isolate and was therefore excluded as a transmission event. No other possible transmission episodes occurred in the households where the index patient isolate was not available for typing.
S. aureus colonization and transmission
Colonization patterns were different in the studies. Table 3 presents the distribution of S. aureus colonization in index patients, S. aureus colonization in non-index household members, and S. aureus transmission by study. In the NL study, the index case had a high level of colonization with MRSA (62%) compared to the other studies. Of non-index household members, the NL study had a low level (20%) of colonization with methicillin-susceptible S. aureus (MSSA) compared to the other studies. In the pooled data, 67 households (23%) had evidence of transmission of the clinical isolate. Despite the different levels of colonization in the studies, levels of transmission of the clinical isolate were not different across the studies (US: 27%, n = 37; NL: 21%, n = 13; AU: 18%, n = 17; P = 0·266).
Table 3. Colonization and transmission of Staphylococcus aureus by study

MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; HHM, household member.
P value refers to test for heterogeneity between studies. χ 2 tests were used for categorical variables and ANOVA for continuous variables.
* Indicates standardized residuals are >1·96 or <–1·96, thus indicating which specific study is accounting for observed heterogeneity between studies.
Risk factors for household transmission
Bivariate analyses assessing risk factors for household transmission of the clinical isolate were conducted in each study. In the US study, the index patient being colonized with the clinical isolate at the time of the home visit was positively associated with household transmission of the clinical isolate (P = 0·02). In the AU study, household size was positively associated with household transmission of the clinical isolate (P = 0·04) (see Supplementary Table S1).
The data were pooled to assess risk factors for household transmission of S. aureus across studies and strain types. Table 4 presents the results of these analyses. In bivariate models, being born in country, the index patient being colonized with the clinical isolate at the time of the home visit, household size, and percent of children in the household were positively associated with transmission at P < 0·20. An increased time interval between the sampling of the clinical isolate and colonization in the household was negatively associated with transmission at P < 0·20. These variables were selected for multivariate analyses.
Table 4. Bivariate analyses of index patient and household (HH) characteristics by HH transmission of the clinical isolate among pooled data
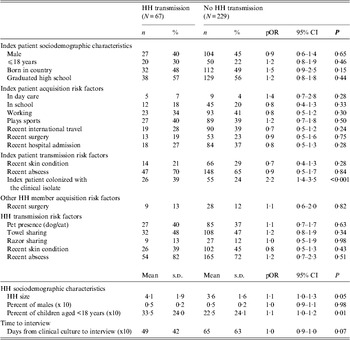
pOR, Prevalence odds ratio; CI, confidence interval.
In multivariate analyses using pooled data, the index patient being colonized with the clinical isolate at the time of the home visit (pOR 2·18, 95% CI 1·37–3·48, P = 0·001) and the percent of household members that were children aged <18 years (pOR 1·13, 95% CI 1·03–1·24, P = 0·008, for a 10% increase) were both independently associated with household transmission of the clinical isolate (see Table 5).
Table 5. Multivariate analyses of index and household (HH) characteristics by HH transmission of the clinical isolate in each study and pooled data
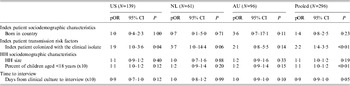
pOR, Prevalence odds ratio; CI, confidence interval.
DISCUSSION
We examined heterogeneity of the spread of CA-MRSA within households of infected cases across multiple geographical regions and attempted to identify common risk factors for household transmission. A diverse set of household characteristics, colonization patterns, and clonal lineages accounting for the burden of S. aureus infections in each study were observed. Despite this variability, frequency of household CA-MRSA transmission was similar across studies and we identified several common risk factors for transmission within the household. Nasal colonization of the index patient with the clinical isolate and the percent of children in the household were risk factors for CA-MRSA household transmission.
There was great diversity in clinical strain types across studies. The US study was dominated by the epidemic strain MRSA t008, which has emerged as the most common cause of CA-MRSA infections in North America [Reference David and Daum4]. While MRSA t008 was present in the NL and AU studies, most infections were caused by a diverse set of non-t008 clonal lineages. It has been speculated that, without adequate control to halt its spread, MRSA t008 will continue to gain ground around the world as the predominant epidemic clone [Reference Tenover and Goering35]. However, our findings suggest, that household transmission of the clinical isolate is equally likely to occur across study populations, regardless of the presence of an epidemic clone, which argues against using strain-targeted intervention strategies [Reference Knox9].
Household colonization patterns also differed between studies. Specifically, the NL study had a higher proportion of index patients colonized with MRSA and a lower proportion of households with a non-index member colonized with MSSA. We can only speculate as to the reason for these differences. They may be a reflection of distinct treatment practices where patients in NL are less likely to be cleared of nasal colonization by the time of the home visit. On the other hand, higher levels of colonization in index patients compared to other household members in the NL study may emphasize the importance of initial index acquisition factors in this setting, vs. subsequent spread in members of a shared household once MRSA has been introduced. Despite these differences in the epidemiology of S. aureus across studies, and the aforementioned differences in biology, overall levels of CA-MRSA household transmission did not differ between studies, and were similar to other reports in the community setting [Reference Wagenvoort5–Reference Huijsdens8].
Our analyses indicate that certain risk factors are correlates of intra-household CA-MRSA transmission. Colonization of the index patient with the clinical isolate was a risk factor for the colonization of other household members with the identical clone. Failure to eliminate colonization in a household member could serve as a potential reservoir for ongoing household transmission, increasing the risk of recurrent colonization and infection even after antibiotic treatment [Reference Jones16–Reference Cook18]. These findings suggest that strategies to limit S. aureus transmission in the community setting should consider decontamination of infected individuals and their household contacts [Reference Bocher36]. Alternatively, given that multiple strain types can often be found colonizing index patients and their household contacts after an initial infection, which has been observed in this study and others [Reference Miller28], another potential solution for interrupting S. aureus transmission and subsequent infection could be re-colonization strategies focused on not inadvertently eliminating less pathogenic S. aureus strains and thus disrupting commensal flora [Reference Iwase37]. Further research into this area is needed.
Our analyses also identified the presence of children in the household as a risk factor for S. aureus transmission. While the effect estimate for this finding was small, it was statistically significant, and indicates that the risk of transmission increases linearly with the proportion of children in the household. A higher proportion of children may represent an elevated level of physical contact between household members. In a previous study conducted in a sample of households with children who had a CA-MRSA infection, bathing the child was identified as a risk factor for the spread of the clinical isolate to the other household members [Reference Nerby24]. The presence of young children was also identified as a risk factor for transmission of all S. aureus by the US research group in a case-control study of households with and without S. aureus infection [Reference Knox9]. However, neither children (5–18 years) nor young children (<5 years) with CA-MRSA infections were more likely to transmit the clinical strain to other household members compared to infected adults (19–65 years) in another multi-site study [Reference Miller28]. While our findings suggest that efforts to limit the spread of CA-MRSA should take into consideration host factors and the composition of infected cases' households, particularly with regard to the presence of children, further research is still needed.
The time from culture to interview was not found to be an independent predictor of household transmission, although we did observe a trend (P = 0·054) that transmission was less likely to be identified when more time passed between the initial infection and the home visit. This near-finding is in accordance with the results of a previous a study that showed that colonization of non-index household members decreased over time, and that colonization was more likely to persist when multiple members of a household were colonized [Reference Lautenbach13]. This is further supported by another study that showed that MRSA carriage can often be fleeting and that minimizing the time between infection and sampling can increase the odds of identifying a positive isolate [Reference Morris38]. Because of the far longer and more variable time from clinical culture to interview in the AU study vs. the two other studies in our analyses (US and NL), we also ran the same staged analyses excluding the AU cases and achieved notably similar results, with only the index patient being colonized with the clinical isolate at the time of the home visit and the percent of children in the household being independent predictors of household transmission of the clinical isolate in multivariate analyses.
The results from the individual studies when compared to the findings from the pooled analyses were, overall, very similar. Effect estimates from the individual studies are almost all in the same direction and only vary in magnitude, so in effect the pooled results resemble a summary of the individual results. Of note, the pooled results are able to achieve statistical significance in many instances where the results from the individual studies do not, thus highlighting the increased statistical power achieved by pooling data from multiple studies. This may be of particular use as CA-MRSA infections remain relatively rare in non-epidemic settings [Reference David and Daum4], and thus studies often struggle to identify enough participating cases to adequately explore relevant research questions.
There are certain limitations to the current study. First, this is a retrospective, observational study that uses a proxy variable as evidence of probable household transmission. Therefore, neither the directionality nor the source of transmission may be ascertained and the shared strains in household members potentially indicate a shared exposure. Second, our analyses were limited to variables shared across all studies. There were other potential risk factors that were not assessed because they were not included in all three studies or were not measured uniformly. These include environmental contamination and poultry consumption, which were associated with S. aureus carriage in previous analyses using data from these studies [Reference Knox9, Reference Uhlemann10, Reference van Rijen25]. Additionally, these three studies did not use uniform time periods for assessing previous risk factors (US: 6 months; NL and AU: 1 year) and these data were unable to be harmonized. Third, different culture techniques were used across studies. Ideally, uniform methods would be used across geographical locations to maximize comparability. Last, this study did not assess the impact of colonization of other body sites as the anterior nares was the only body site sampled in all three studies, even though this has emerged as a common feature of CA-MRSA carriage [Reference Miller28, Reference Miller32, Reference Lee39]. Underestimation of S. aureus colonization may, in turn, underestimate household transmission. Despite these limitations, our pooled analysis benefits from a large, diverse sample size resulting in strong analytical power and increased generalizability.
Our study identifies shared features of CA-MRSA household transmission despite geographical difference in strain profiles. The spread of CA-MRSA in households increases the likelihood of re-infection in its members [Reference Uhlemann10, Reference Jones16–Reference Cook18]. Furthermore, the ability of infectious S. aureus strains to persist in households increases the likelihood that they will spread through the community [Reference Zafar11–Reference Ho14]. Our findings suggest that decontamination strategies targeting the household unit may be effective in reducing the transmission of S. aureus colonization and infection in the community setting. Such interventions appear applicable across diverse, international patient populations. Prospective, multicentre studies are needed to further define the transmission patterns of this prevalent and highly pathogenic organism.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814000983.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (grant nos. AI077690, AI090013); The Netherlands Organisation for Health Research and Development (ZonMw); and the National Health & Medical Research Council (grant no. 509304).
DECLARATION OF INTEREST
None.











