Introduction
Staphylococcus aureus is among the most common causes of bloodstream infection (BSI) and is widely recognised as the most important cause of BSI-associated death [Reference Uslan1, Reference Laupland2] being associated with a high mortality rate, presentation of persistent bacteraemia and frequent spread to distant foci [Reference Khatib3, Reference van Hal4]. Prior to the mid-1990s, methicillin-resistant S. aureus (MRSA) was almost exclusively a healthcare-associated pathogen (HA-MRSA) and infections in the community were nearly always caused by methicillin-susceptible strains (MSSA). However, the end of the twentieth century saw the emergence of community-associated MRSA (CA-MRSA) strains in patients without traditional epidemiological risk factors for MRSA infection and/or colonisation [Reference David and Daum5]. Subsequently, CA-MRSA has spread rapidly into hospital settings and has recently begun replacing pre-existing HA-MRSA strains in healthcare facilities [Reference David and Daum5–Reference Brady7]. This spread of several different CA-MRSA clones has complicated the epidemiological picture and posed a new challenge for infection prevention and control [Reference Cho and Chung8].
Previous studies have suggested that the delay in initiation of anti-MRSA therapy adversely affects the outcome of patients with MRSA bacteraemia, especially those with high Pitt Bacteraemia Scores [Reference Wi9, Reference Paul10] and that vancomycin is inferior to beta-lactams for the treatment of MSSA bacteraemia [Reference Kim11]. As a result, risk factors associated with MRSA infection have been highlighted to optimise antibiotic use when initiating empirical antibiotics against S. aureus [Reference Chalmers12, Reference Aliberti13]. A previous study showed that a patient history of MRSA infection or colonisation, recurrent skin infection and severe community-acquired pneumonia were independent predictors of community-acquired MRSA pneumonia [Reference Aliberti13]. However, a systematic review of 27 published studies showed that previous admission to hospital and antibiotic use were the only commonly examined risk factors associated with MRSA infection at admission, albeit with varying definitions [Reference Forster14]. The authors emphasised that clinicians and health system planners face many challenges in identifying individuals predisposed to MRSA infection [Reference Forster14]. Therefore, it is important to examine the predictors of MRSA infection and to determine whether the associated factors have changed in recent years.
We compared the clinical features of MSSA and MRSA bacteremia cases with a view to identify factors predictive of MRSA infection. In addition, we evaluated the impact of methicillin resistance on mortality in these patients.
Methods
Patients and study design
The study data were obtained from databases for nationwide surveillance of bacteraemia. All patients with positive blood cultures for S. aureus were enrolled at 9 secondary- or tertiary-care hospitals through prospective surveillance studies during two periods from June to September 2011 and June to September 2012. Duplicate isolates were excluded from the analysis. For outcome measures, patients with polymicrobial bacteraemia were also excluded. We identified 371 consecutive patients with confirmed S. aureus bacteraemia and patient information was entered on a standardised case report form. The data collected included demographic information, co-morbid diseases and conditions, location of bacteraemia onset, shock at presentation, source of bacteraemia, antibiotic use and surgery within the preceding 90 days and appropriateness of antimicrobial treatment. Echocardiographs were performed for all patients with persistent S. aureus bacteraemia of more than 7 days duration. The study was approved by the Institutional Review Board of Samsung Medical Center and the local review boards.
All isolates were confirmed as S. aureus by standard methods and tested for antimicrobial susceptibility at each participating hospital using an automated system for the modified broth microdilution method (Vitek; bioMérieux, Durham, NC, USA, or Microscan; Microscan Systems, Renton, WA, USA) according to the recommendations of the Clinical and Laboratory Standards Institute (2010) [15].
Definitions
Clinically significant bacteraemia was defined as at least one positive blood culture together with clinical features consistent with systemic inflammatory response syndrome. Catheter-related BSI was defined as growth of >15 colony-forming units from the catheter tip in a semiquantitative culture or growth of S. aureus from a blood sample drawn from a catheter hub at least 2 h before MRSA was obtained from a peripheral venous blood sample. Infective endocarditis was defined according to the modified Duke criteria [Reference Li16]. Neutropaenia was defined as an absolute neutrophil count of <500/mm3. Isolates defined as ‘community-onset’ were cultured in the outpatient setting and within 48 h of hospital admission while ‘hospital-onset’ isolates were classified as obtained 48 h after hospitalisation. Community-onset infections were further categorised as community-acquired (CA) or HA. HA infections were defined as previously described [Reference Friedman17]. Antibiotic use in preceding 90 days was collected by chart review and through interview with patients and prescribers; prescribed antibiotics was further classified into four classes; glycopeptides, β-lactams, fluoroquinolones and others. A history of MRSA was determined through a review of each hospital's clinical microbiology record and included both asymptomatic MRSA colonisations and infections in the previous 90 days. The severity of underlying disease was classified according to the McCabe and Jackson criteria: rapidly fatal when death was expected within days or weeks; ultimately fatal when death was expected within months or years; and non-fatal when death was not expected [Reference McCabe18]. In addition, Pitt Bacteraemia Scores were determined for all patients [Reference Paterson19]. Appropriate antibiotic use was defined as the administration of antibiotics, to which the isolated organism was susceptible in vitro, within 24 h after the acquisition of blood culture samples. Persistent bacteraemia was defined as persisting for ⩾3 days after initiation of appropriate antibiotic treatment. The outcome measure was the 30-day all-cause mortality, beginning from the date of the index culture. Deaths occurring after discharge but within the 30-day interval were captured using the National Health Insurance system of the Republic of Korea, which records all deaths.
Statistical analysis
Discrete data are presented as frequencies and percentages and continuous variables are summarised as the mean±standard deviation after the normality of data was tested using the Shapiro–Wilk normality test. The chi-square (χ 2) test or Fisher's exact test was used to compare categorical variables as appropriate and two-sample t-test was used to compare continuous variables. To identify predictors of methicillin resistance in patients with S. aureus bacteraemia, a multivariate logistic regression analysis was used to control for the effects of confounding variables and to identify predictors of mortality, a multivariate Cox proportional hazards regression model was used to control for the effects of confounding variables. Variables with P value <0.05 were included in the multivariate analyses. The Hosmer–Lemeshow test was used for goodness of fit for logistic regression models. All P values were 2-tailed and P < 0.05 was considered significant. All analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients with S. aureus bacteraemia
A total of 371 patients met the case definition of S. aureus bacteraemia; MSSA was isolated from 155 patients (41.8%) and MRSA from 216 patients (58.2%). MRSA accounted for 42.2% of community-onset and 74.5% of hospital-onset cases. The clinical characteristics and outcomes of patients with S. aureus bacteraemia are summarised in Table 1. Significant differences in clinical and epidemiological characteristics between MRSA and MSSA were found for several variables; hospital-onset origin (P < 0.001), surgery in the preceding 90 days (P < 0.001), antibiotic use in the same period (P < 0.001), history of MRSA infection (P = 0.002), underlying cerebrovascular disease (P = 0.005) or haematologic malignancy (P = 0.035), undergoing immunosuppressive therapy (P = 0.030), presentation with central venous catheterisation (P < 0.001) or percutaneous drainage (P = 0.001), presentation with acute renal failure (P < 0.001), primary bacteraemia (P = 0.049) and catheter-related BSI (P = 0.030) were risk factors for having MRSA bacteraemia. The multivariate analysis showed that hospital-onset (odds ratio (OR) 2.82, 95% confidence interval (CI) 1.62–4.91, P < 0.001) and any antibiotic use in the preceding 90 days (OR 2.47, 95% CI 1.41–4.34, P = 0.002) were independent predictors for MRSA bacteraemia. The goodness of fit of the final logistic regression model appeared to be satisfactory (Hosmer-Lemeshow statistic, χ 2 = 5.627, P = 0.689). The frequency of inappropriate empirical therapy was higher in patients with MRSA than MSSA bacteraemia (56.5% vs. 2.6%, P < 0.001), but 30-day mortality rates did not differ between the two groups (20.9% vs. 21.5%, P = 0.828).
Table 1. Clinical predictors and outcomes of methicillin resistance bacteraemia in 371 patients with Staphylococcus aureus bacteraemia
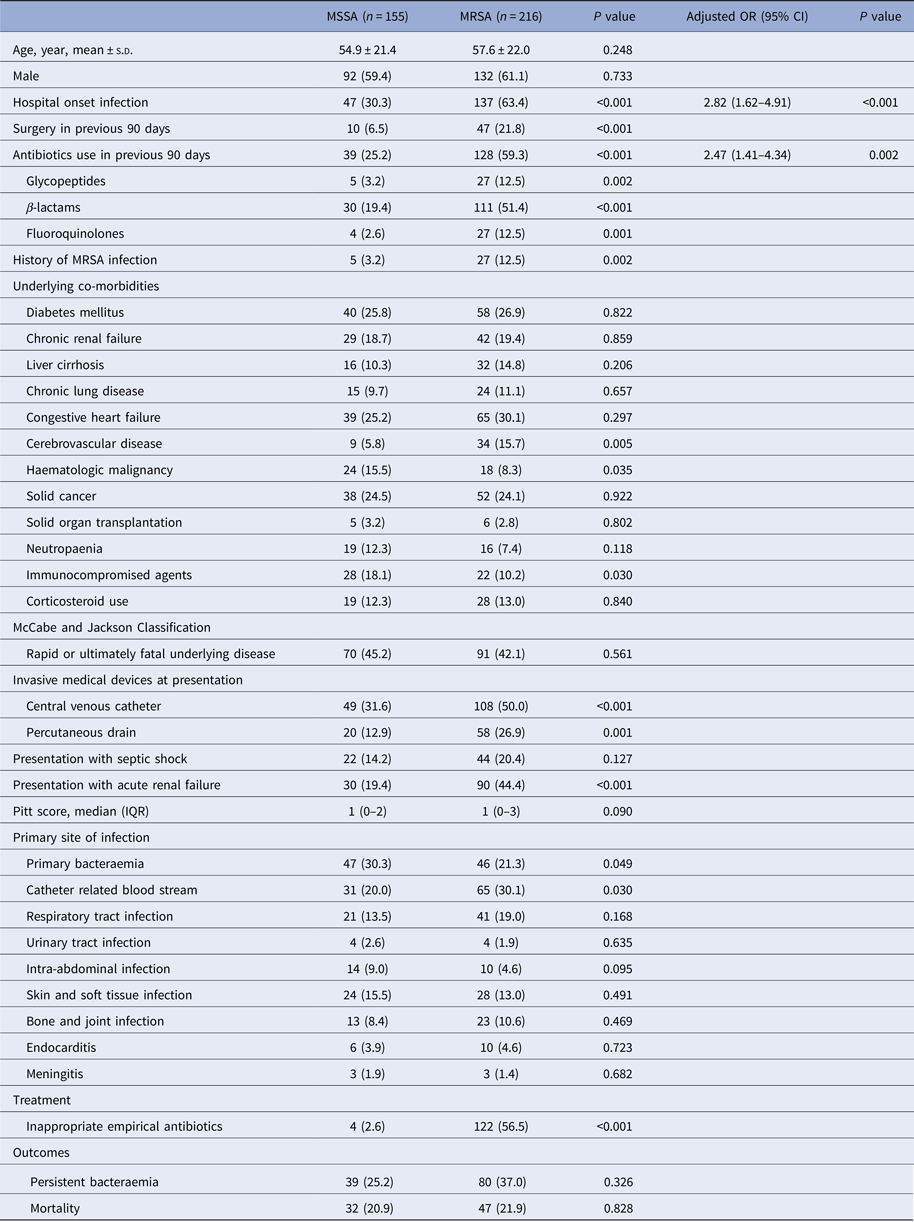
MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; OR, odds ratio; CI, confidence interval; s.d., standard deviation; IQR, interquartile range.
Hosmer-Lemeshow goodness of fit statistic χ 2 = 5.627, P = 0.689.
Data are n (%) unless otherwise stated.
Clinical characteristics and outcomes of MRSA according to primary acquisition site
The clinical characteristics and outcomes of MRSA among S. aureus bacteraemia patients according to acquisition site are presented in Table 2. Surgery in the previous 90 days (P = 0.007), antibiotic use in the same period (P = 0.005) and bone and joint infection (P = 0.032) were risk factors for CA-MRSA bacteraemia. By multivariate analysis only prior antibiotic use (OR 2.04, 95% CI 1.01–4.14, P = 0.047) was an independent predictor of MRSA in community-onset cases. Prescribed β-lactams was the only antibiotic class associated with having MRSA bacteraemia among prior antibiotic uses in both the multivariate analysis and in bivariate testing. The goodness of fit of the final logistic regression model appeared to be satisfactory (Hosmer-Lemeshow statistic, χ 2 = 2.701, P = 0.259). On the other hand, for hospital-onset cases, several risk factors were more prevalent in MRSA than MSSA bacteraemia including: surgery in previous 90 days (P = 0.040), antibiotic use in the same period (P < 0.001), underlying diabetes (P = 0.017), underlying cerebrovascular diseases (P = 0.008) or haematologic malignancy (P = 0.006), neutropaenia (P = 0.035), use of immunosuppressive agents (P = 0.001), central venous catheterisation (P = 0.030) and percutaneous drainage at presentation (P = 0.021), presentation with septic shock (P = 0.040) or acute renal failure (P < 0.001). Prior use of any antibiotic (OR 2.25, 95% CI 1.01–5.01, P = 0.047) was also the only independent predictor of HA-MRSA bacteremia in multivariate analysis. The goodness of fit of the final logistic regression model in hospital-onset population appeared to be satisfactory (Hosmer-Lemeshow statistic, χ 2 = 6.095, P = 0.637).
Table 2. Clinical predictors and outcomes of patients with Staphylococcus aureus bacteraemia stratified by acquisition site

MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; s.d., standard deviation; IQR, interquartile range.
Hosmer-Lemeshow goodness of fit statistic for community-onset infection χ 2 = 2.701, P = 0.259.
Hosmer-Lemeshow goodness of fit statistic for hospital-onset infection χ 2 = 6.095, P = 0.637.
Data are n (%) unless otherwise stated.
Predictors of 30-day mortality in patients with S. aureus bacteraemia
The multivariate analysis of potential risk factors associated with 30-day mortality of cases is shown in Table 3. Variables with P-value of <0.05 in the univariate analysis (i.e. age, liver cirrhosis, solid cancer, rapidly or ultimately fatal disease, central venous catheterisation at presentation, septic shock, acute renal failure, Pitt Bacteraemia Score, respiratory, or skin and soft tissue infection, endocarditis and persistent bacteraemia) were included in the subsequent multivariate analysis. A Cox proportional hazards model revealed that of the above factors, age (hazard ratio (HR) = 1.03, 95% CI 1.01–1.04; P = 0.001), liver cirrhosis (HR = 2.21, 95% CI 1.24–3.97, P = 0.008), rapidly or ultimately fatal disease (HR = 2.38, 95% CI 1.46–3.89, P = 0.001), septic shock (HR = 2.14, 95% CI 1.22–3.78; P = 0.008), Pitt Bacteraemia Score (HR = 1.14, 95% CI 1.05–1.23, P = 0.002), skin and soft tissue infection (HR = 0.28, 95% CI 0.09–0.90, P = 0.032) and persistent bacteraemia (HR = 2.84, 95% CI 1.76–4.56; P < 0.001) were independent predictors of 30-day mortality. Methicillin resistance (HR = 1.01, 95% CI 0.66–1.53; P = 0.972) was not a predictor of mortality in these patients and remained non predictive of mortality after stratifying according to acquisition site in both community-onset (HR = 0.96, 95% CI 0.52–1.77; P = 0.889) and hospital-onset cases (HR = 1.06, 95% CI 0.54–2.07; P = 0.862) (Table 4).
Table 3. Variables associated with 30-day mortality in 371 patients with Staphylococcus aureus bacteraemia
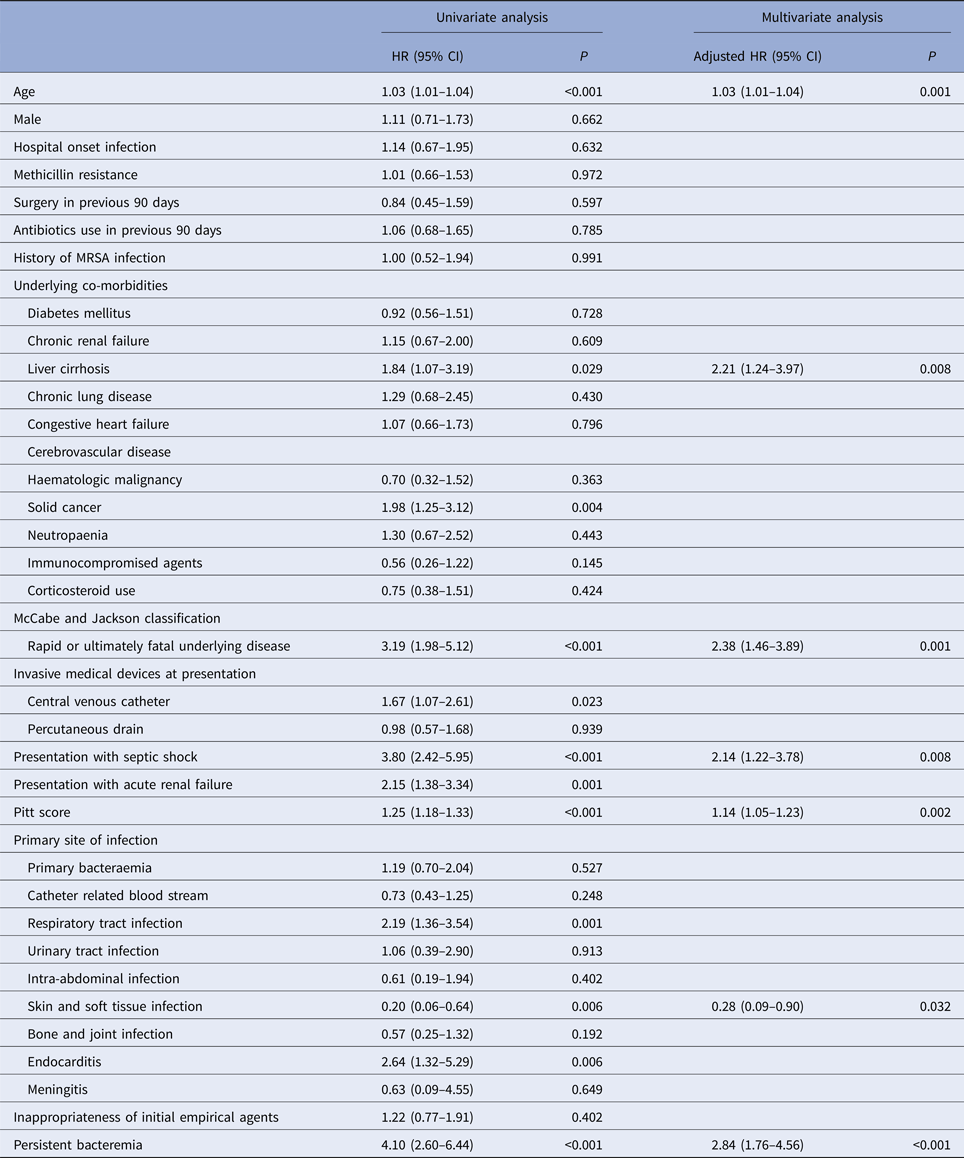
HR, hazard ratio; MRSA, methicillin-resistant S. aureus.
Table 4. Variables associated with 30-day mortality in 371 patients with Staphylococcus aureus bacteraemia stratified by acquisition site
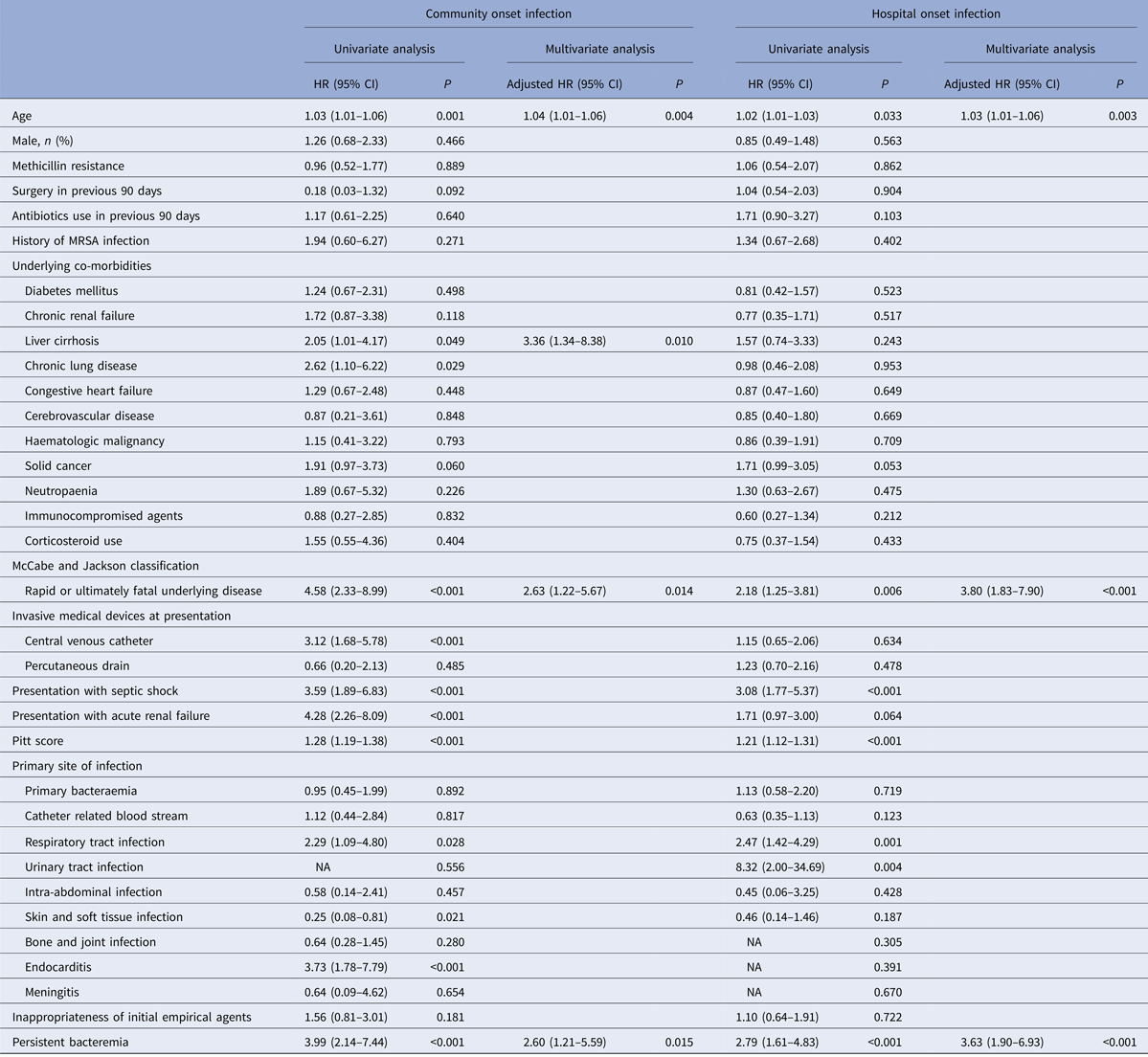
HR, hazard ratio; MRSA, methicillin-resistant S. aureus.
Discussion
Our study found no significant differences in clinical outcome between bacteraemic patients infected with MRSA vs. MSSA. However, MRSA patients were more likely to have acquired the infection in hospital and received antibiotics in the preceding 90 days and this was the only marker predictive of methicillin-resistance. Inappropriate empirical therapy was more frequently prescribed for MRSA bacteraemia but the 30-day mortality did not differ between the two groups.
The epidemiology of S. aureus has undergone significant changes over the past three decades. Initially, HA-MRSA infection was thought to be primarily a HA disease; however, several CA-MRSA strains have emerged in the community some of which have recently spread into healthcare settings [Reference David and Daum5–Reference Brady7]. In addition, a recent study from Chicago, USA showed that a possible role reversal had occurred for MSSA and MRSA strains as the former were predominantly HA and many MRSA patients no longer had recent exposure to the healthcare setting [Reference David20]. Therefore, it is important to examine the factors that may be associated with MRSA infection. Miller et al. [Reference Miller21] showed from a prospective investigation of consecutive hospitalised patients with S. aureus infection in Los Angeles, that clinical and epidemiological risk factors did not reliably distinguish between MRSA and MSSA. Similar findings were reported from a study of S. aureus infections among military veterans except that methicillin resistance was higher in subjects who had a prior MRSA infection or stay in a long-term care facility [Reference McCarthy22]. Likewise, a cross-sectional study from Colombia found that most clinical and epidemiological factors evaluated did not allow discrimination of MRSA from MSSA infected patients with the exception of a history of MRSA infection, prior antibiotic therapy and stay in children's day care centres [Reference Jimenez23]. These factors are corroborated by the few clinical differences found in our study between MRSA and MSSA patients, with prior antibiotics being the only independent predictor of both CA- and HA-MRSA bacteraemia. In community-onset S. aureus cases, only prior β-lactam therapy was a risk factor for having MRSA, as opposed to all classes of antibiotics for hospital onset MRSA. In another study, prior exposure to the carbapenems appeared to have a gradient effect for the emergence of methicillin resistance [Reference Arias-Ortiz24].
Other established risk factors [Reference Lowy25, Reference Brumfitt and Hamilton-Miller26], including history of MRSA infection, residing in a long-term care facility with MRSA infections, recent surgery and indwelling percutaneous medical devices and catheters, failed to differentiate MRSA from MSSA infection in our study. Taken together with other studies, these results support the view that MRSA and MSSA bacteraemic infection share most clinical characteristics. This might be explained by the striking increase of CA-MRSA infections in many countries and their dissemination in to healthcare settings.
Consistent with previous studies [Reference Kaplan27, Reference Fridkin28], the clinical spectrum of disease caused by MRSA appears to be similar to that of MSSA in the community. However, Lee et al. [Reference Lee29] suggested that bone and joint infection was a predictor of CA-MRSA bacteraemia in Korea and identified a specific clone, ST72-SCCmec IV/IVA, which predominated in primary bone and joint infections that progressed to bacteraemia. In our study, bone and joint infections were significantly more prevalent in patients with MRSA than those with community-onset MSSA but statistical significance was lost after adjusting for prior surgery and antibiotic use. The predominance of bone and joint infections in patients with CA-MRSA bacteraemia warrants further investigation.
Many previous studies evaluating the outcomes of MRSA bacteraemia have shown conflicting results [Reference Yilmaz30–Reference Mylotte and Tayara33]. Some have reported significant association of MRSA with mortality [Reference Yilmaz30, Reference Blot32], while others have not [Reference Cosgrove31, Reference Mylotte and Tayara33–Reference Yaw, Robinson and Ho35]. In the Cox regression model, we could not link MRSA bacteraemia with higher mortality than that with MSSA and after stratifying by acquisition site, methicillin resistance was still not a significant predictor of mortality. Outcomes of MRSA infections could also be made worse due to confounding associations with other prognostic factors. Almost all relevant studies have shown that infections due to MRSA occur in sicker patients [Reference Yaw, Robinson and Ho35, Reference Cosgrove36], suggesting that differences in mortality may, at least in part, be attributable to the patients’ underlying illnesses. Our study population showed few significant clinical differences between MRSA and MSSA bacteraemia and this was reflected in their similar mortality rates. Indeed, previous studies of patient outcomes with MRSA bacteraemia also linked this to a longer hospital stay and not with mortality [Reference Lodise and McKinnon37, Reference Reed38].
We have shown that patients with MRSA bacteraemia were more likely to receive inappropriate empirical antibiotic therapy but this was not an independent predictor of mortality. Others have reported conflicting findings on this issue [Reference Gasch39–Reference Khatib44]. Interestingly, three studies conducted in Asia found that this factor did not have a detrimental effect on mortality in MRSA bacteraemic patients [Reference Kim43, Reference Fang45, Reference Yoon46]. Yoon et al. [Reference Yoon46] in Korea considered that an initial delay in the use of definitive antibiotics did not necessarily prejudice the clinical outcomes of patients with HA-MRSA bacteraemia and recommended the prudent use of glycopeptides as empirical therapy in the context of increasing antibiotic resistance. Similarly, in Taiwan, Fang et al. [Reference Fang45] did not support the view that earlier empirical use of glycopeptide therapy reduces mortality in patients with HA-MRSA bacteraemia and, likewise, no significant difference in MRSA-related mortality was evident between patients who had received an appropriate or inappropriate empirical regimen [Reference Kim43].
Some limitations of this study are noted. First, only measured variables were controlled and some additional confounding factors were not accounted for such as prior residence in a long-term care facility, or receipt of hemodialysis and hospitalisation, which are often cited in similar studies as contributory factors differentiating between MRSA and MSSA patients. Second, the failure to show a significant difference in mortality according to empirical antibiotic therapy may be a consequence of the limited sample size; third, and we chose to define appropriate antibiotic use based on in vitro susceptibility of the recovered isolate and did not take account of the fact that different agents differ in clinical outcomes such as the reported inferiority of vancomycin to beta-lactams for the treatment of MSSA bacteraemia [Reference Kim11, Reference Khatib44]. Finally, the absence of molecular analysis did not allow investigation of the clonal background which might have impacted on mortality given the reported associations of, for example, strain lineages CC22 and endocarditis, [Reference Miller47], CC8 and vancomycin resistance [Reference Holmes48] and CC5 or CC30 and haematogenous complications [Reference Fowler49]. The predominant CA-MRSA clone in Korea, ST72-SCCmecIV, was shown to be independently associated with lower mortality compared with ST5-SCCmecII [Reference Park50]; furthermore, the lack of virulence markers including staphylococcal superantigen genes, may play a role in the lower virulence of ST72-SCCmecIV strain [Reference Park50]. Further clonal analysis of both MRSA and MSSA strains is therefore necessary to explore further any associations of strain type with mortality.
In conclusion, we found no definitive clinical or epidemiological risk factors which could distinguish MRSA from MSSA in bacteraemic patients with the exception of the previous use of antibiotics for having MRSA bacteraemia in such patients regardless of its acquisition site. MRSA bacteraemia was not associated with higher risks of mortality. Finally, the prudent use of glycopeptide treatment of patients at risk for invasive MRSA infections should be emphasised in order to stem the tide of increasing resistance to these highly effective agents.
Conflict of interest
None.









