Introduction
Major depressive disorder (MDD) is a pervasive mental health condition. In 2015, the National Institute of Mental Health reported that approximately 16 million adults in the USA had at least one major depressive episode (MDE) in the past year.1 Depression and the timing of MDEs have been established as risk factors for suicide-related behavior.Reference Oquendo, Perez-Rodriguez and Poh2 Recurrent suicidal ideation has been established as a diagnostic criterion for MDD.3 In addition, a combination of individual, relationship, community, and societal factors, including stressful life events, family history of suicide, mental disorders, prolonged stress, and substance use problems, can contribute to the risk of suicide-related events.Reference Zalsman, Hawton and Wasserman4–6 In an effort to mitigate the symptoms of MDD, antidepressants have been widely used to effectively treat psychiatric mood disorders, including MDD.Reference Castelpietra, Gobbato and Valent7 From 2011 to 2014, 12.7% of people in the USA over 12 years of age reported taking antidepressants within the past 30 days, and 25% of those patients reported taking antidepressants for 10 years or more.Reference Pratt, Brody and Gu8 When antidepressant usage outside of the USA is considered, worldwide use of antidepressants has risen steadily between 2000 and 2014.Reference Gautam9
Despite the widespread use of antidepressants, the debate persists that they can be associated with an increased risk of suicide-related events in some patients. In 2004, the US Food and Drug Administration (FDA) Advisory Committee on Psychopharmacologic Drugs determined that warning labels for all antidepressants should include information about an increased risk of suicidality in children and adolescents with MDD,10, Reference Hammad, Laughren and Racoosin11 which was extended in 2007 to include young adults ≤24 years of age.12, Reference Stone, Laughren and Jones13 However, the FDA reviewed 207 short-term randomized clinical trials in adult patients with MDD or various anxiety disorders and did not detect an increased risk of completed suicide in either the antidepressant or the placebo groups.Reference Hammad, Laughren and Racoosin14 This finding conforms with several additional meta-analyses using different measures of depression (Hamilton Depression Rating Scale [HAM-D], Montgomery–Åsberg Depression Rating Scale [MADRS]); none of these analyses showed an increased risk of suicide-related events in adult patients with MDD and generalized anxiety disorder (GAD) in response to antidepressant usage.Reference Acharya, Rosen and Polzer15–Reference Thase, Gommoll and Chen21 Yet, other studies have indicated a reduction in the risk of suicide in MDD patients because of antidepressant therapy.Reference Castelpietra, Gobbato and Valent7, Reference Kasper, Montgomery and Moller22–Reference Cheung, Aarts and Noordam24
The FDA guidance recommends prospective evaluation of treatment-emergent suicide-related events in clinical trials for psychotropic medications with an instrument that directly classifies them into relevant categories. The Columbia-Suicide Severity Rating Scale (C-SSRS), a scale based on the Columbia Classification Algorithm of Suicide Assessment, was developed to measure suicidal ideation (suicide planning) or behavior (suicidal attempts) in a hierarchical and time-sensitive manner and is one such assessment instrument that is frequently used in clinical trials.Reference Posner, Brown and Stanley25
Vortioxetine is an approved antidepressant for adults with MDD in the USA,26 the European Union, and other countries worldwide.27 It has a unique mechanism of action thought to be related to its multimodal activity (as a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist; a 5-HT1B receptor partial agonist; a 5-HT1A receptor agonist; and an inhibitor of the serotonin [5-hydroxytryptamine, 5-HT] transporter [5-HTT or SERT]).Reference Bang-Andersen, Ruhland and Jorgensen28–Reference Connolly and Thase30 Vortioxetine’s antidepressant efficacy, safety, and tolerability for doses from 5 to 20 mg/day in patients with MDD have been shown in a global clinical development program.Reference Baldwin, Chrones and Florea31
These post hoc analyses evaluated the potential risk of suicidal ideation and behavior in adult patients with MDD treated with vortioxetine using pooled data from 10 randomized, placebo-controlled, short-term studies (6 or 8 weeks)Reference Jain, Mahableshwarkar and Jacobsen32–Reference Katona, Hansen and Olsen41 and 3 open-label, long-term extension studies (52 weeks)Reference Fillipov and Christens20, Reference Alam, Jacobsen and Chen42, Reference Jacobsen, Harper and Chrones43 based on C-SSRS scores (measured in 7 of 10 randomized, placebo-controlled, short-term studies and 3 open-label, long-term extension trials) and treatment-emergent adverse events (TEAEs) data.
Methods
This post hoc analysis was conducted using data from 10 randomized, placebo-controlled, short-term trials (6 or 8 weeks), herein referred to as the “short-term pool,” and 3 open-label, long-term extension trials (52 weeks), herein referred to as the “long-term pool.” Integrated safety data trials were analyzed for all patients receiving at least one dose of study drug, and patients were analyzed according to the treatment (dosage) received. Two study pools were generated by analyzing applicable phase II and III studies in adults aged 18–75 years. Results for active references, duloxetine and venlafaxine, were included and analyzed where appropriate. Only one study included venlafaxine as active reference, and the venlafaxine cohort was excluded in these analyses given the small patient number in this group.Reference Alvarez, Perez and Dragheim39 Handling of safety data and safety analysis was harmonized across databases to ensure consistent reporting. Suicide-related events were assessed using C-SSRS data from 7 short-term trials and TEAEs from 10 short-term trials (Table 1). C-SSRS data were only available for 7 of 10 short-term trials. C-SSRS data were not collected in two short-term trialsReference Alvarez, Perez and Dragheim39, Reference Baldwin, Loft and Dragheim40 or in the case of a third trial, where C-SSRS data were collected differently because of a protocol amendment after enrollment had begun.Reference Katona, Hansen and Olsen41 Both C-SSRS and TEAE data were evaluated in the three open-label, long-term extension trials. All clinical trials used for the pooled analyses are summarized in Tables 1 and 2, and detailed methods for all individual clinical trials have been published previously.Reference Jain, Mahableshwarkar and Jacobsen32–Reference Jacobsen, Harper and Chrones43
TABLE 1. Overview of MDD studies included in the short-term pool

Notes: C-SSRS, Columbia-Suicide Severity Rating Scale; DLX, duloxetine; MDD, major depressive disorder; TEAE, treatment-emergent adverse events; VEN, venlafaxine; XR, extended-release.
Short-term and long-term trials were investigated with a range of doses from 1 to 20 mg; however, therapeutic doses of vortioxetine are 5–20 mg.
* All trials were randomized, double-blind, and placebo-controlled.
TABLE 2. Overview of MDD studies included in the long-term pool*

Notes: C-SSRS, Columbia-Suicide Severity Rating Scale; MDD, major depressive disorder; TEAE, treatment-emergent adverse events.
* All trials were 52-week, open-label extension studies.
Key inclusion criteria for the studies were women and men aged 18–75 years, with the exception of one study in elderly patients ≥65 years, who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD,3 with an MADRS score of ≥22,Reference Mahableshwarkar, Jacobsen and Chen33 ≥26,Reference Henigsberg, Mahableshwarkar and Jacobsen34–Reference Boulenger, Loft and Olsen38, Reference Baldwin, Loft and Dragheim40, Reference Katona, Hansen and Olsen41 or ≥30.Reference Jain, Mahableshwarkar and Jacobsen32, Reference Alvarez, Perez and Dragheim39 Clinical Global Impression-Severity scores of ≥4 were the criteria in only a few trials.Reference Mahableshwarkar, Jacobsen and Chen35–Reference Boulenger, Loft and Olsen38 All participants in the open-label extension studies were required to complete one of the double-blind lead-in trials before enrolling in the open-label studies. All patients who received at least one dose of study drug were included in the safety analysis. Key exclusion criteria prohibited participation by anyone with any diagnosis of psychiatric disorder (mania, bipolar, etc) as defined by the DSM-IV-TR and patients at serious risk of suicide, as defined by a score of ≥5 on item 10 of the MADRS scale.
Flexible dosing for vortioxetine in the open-label pool was between 2.5 and 20 mg.Reference Alam, Jacobsen and Chen42 The short-term pool included treatment groups given between 1 and 20 mg of vortioxetine; however, data from patients dosed with ≤2.5 mg are not included in these analyses because 1 and 2.5 mg are not approved doses. Because the open-label studies allowed flexible dosing, some patients included in the long-term pool could have been dosed with 2.5 mg.Reference Alam, Jacobsen and Chen42
Ethical conduct and informed consent
Studies were approved by the ethics committees for each study site and were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. All participants in each trial provided written informed consent before trial commencement.
Assessment of suicidal ideation and behavior
The potential risk of suicidal ideation and behavior in response to vortioxetine was evaluated on the basis of the C-SSRS and TEAEs (Tables 1 and 2). The C-SSRS is a recommended measure to prospectively monitor the complex occurrence of suicidal ideation and behavior in clinical trials involving any drug being developed for any psychiatric indication, as well as for all antiepileptic drugs and other neurological drugs with central nervous system activity.Reference Posner, Brown and Stanley25, 44 In each trial, two versions of the C-SSRS (“Baseline” and “Since Last Visit”) were administered. The Baseline version was used at the screening visit and assessed lifetime suicidal events, and the Since Last Visit version assessed all suicidal ideation and behavior at the baseline and postbaseline visits.
C-SSRS assessments for each treatment group were based on three key study periods: all prior history, baseline, and during the treatment period. All prior history assessments used the most severe score for each patient from the screening (lifetime assessment) and baseline (pretreatment assessment) visits. Baseline assessments were determined between screening and baseline visits. During the treatment period, assessments were made at each postbaseline visit. Patients were counted only once per evaluation using the most severe score.
C-SSRS-based incidences were categorized as follows: no suicidal ideation or behavior (C-SSRS score of 0), suicidal ideation (C-SSRS score of 1–5), or suicidal behavior (C-SSRS score of 6–9).44 Completed suicides were given a score of 10 (Table 3). C-SSRS data were analyzed for all patients in the safety set who had at least one postbaseline assessment. Shift analyses were used to illustrate categorical changes occurring for each C-SSRS-based incidence score using the most severe postbaseline score during the study period.Reference Klepper and Cobert45
TABLE 3. Severity ordering for C-SSRS scores
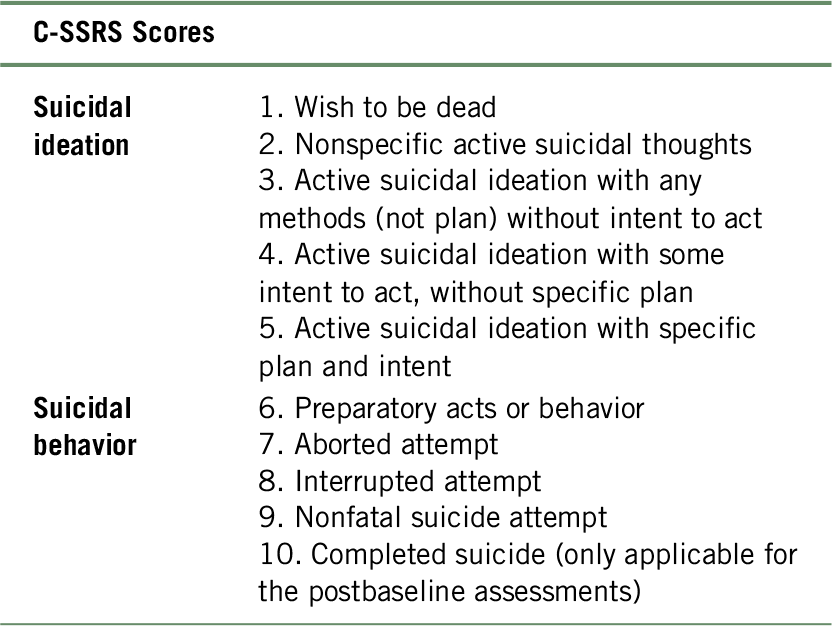
Notes: C-SSRS, Columbia-Suicide Severity Rating Scale.
Suicide-related events
Suicide-related TEAEs were evaluated using Standardized Medical Dictionary for Regulatory Activities (MedDRA) Queries (SMQ) for suicide/self-injury (SMQ code 20000037). Summaries of TEAEs, defined for suicide-related events, were grouped using predefined MedDRA terms, and data were collected during the treatment period similar to C-SSRS data collection. Preferred terms included the following: suicidal ideation, self-injurious behavior, intentional overdose, suicide attempt, and intentional self-injury. The summaries included raw number and percentage of patients with suicide-related events during the treatment period, as well as overall incidence in each group.
Results
Patient characteristics
An overview of the studies included in each pool is provided in Tables 1 and 2. In the short-term pool (10 studies, N = 4990 patients), patient demographics and baseline characteristics were generally similar and well balanced across placebo, all vortioxetine dosage groups (5–20 mg), and the duloxetine group (Supplementary Table 1). The short-term pool included one study that evaluated 5 mg of vortioxetine in elderly patients ≥65 years.Reference Katona, Hansen and Olsen41 Patient demographics and other baseline characteristics in the long-term pool (three studies, N = 1956 patients) were generally similar to those in the short-term pool (Supplementary Table 2).
Suicidal ideation and behavior on C-SSRS
The lifetime histories of suicidal ideation and behavior based on C-SSRS scores were similar across all treatment groups in seven studies that used C-SSRS data for suicide-related events (N = 3614) (Figure 1 and Table 4). Overall, 43.3% of patients in the placebo group (529/1223), 40.3% (180/447) in the duloxetine group, and 43.2% (256/592), 38.6% (173/448), 35.2% (158/449), and 41.1% (187/455) of patients in the 5-, 10-, 15-, and 20-mg vortioxetine treatment groups, respectively, reported any lifetime suicidal ideation or behavior.

FIGURE 1. Suicidal ideation or behavior based on the C-SSRS using all prior history in seven short-term MDD studies. For studies included, see Table 1. Percentages of patients with any suicidal ideation or behavior based on the C-SSRS score of 1–9 using all prior history are shown. All prior history includes events that occurred before administration of the study drug and at screening and baseline visits. Patients were counted once using the most severe event. Patients could have also reported nonsuicidal self-injurious behavior; these nonsuicidal events were not included in determining the most severe event. DLX, duloxetine; MDD, major depressive disorder; n, total number of patients assessed with C-SSRS; PBO, placebo; VOR, vortioxetine.
TABLE 4. Suicidal ideation and behavior based on C-SSRS using all prior history, baseline, and treatment period assessments in the MDD short-term pool*

Notes: C-SSRS, Columbia-Suicide Severity Rating Scale; MDD, major depressive disorder; VOR, vortioxetine.
Values are number of patients (%). All prior history includes events that occurred before administration of study drug and at screening and baseline visits. Baseline period includes events that occurred between screening and baseline visits.
* For studies included, see Table 1. Patients were counted once using the most severe event.
Before treatment, 14.7% of patients reported suicidal ideation in the placebo group (179/1219) compared with 19.6% (116/592), 13.0% (58/446), 11.2% (50/448), and 13.4% (61/454) of patients randomized to 5, 10, 15, and 20 mg of vortioxetine, respectively, and 13.2% (59/447) randomized to duloxetine. One patient in the 5-mg vortioxetine group and one patient in the 20-mg vortioxetine group reported suicidal behavior.
Suicidal ideation and behavior based on C-SSRS in the short-term pool were low and similar across all treatment groups and did not change during the treatment period compared with baseline (Figure 2 and Table 4). There were no completed suicides in any study. In the placebo group, 17% (204/1199) reported suicidal ideation or behavior compared with 19.3% (113/586), 13.5% (60/446), 12.6% (56/445), and 15% (67/447) for the 5-, 10-, 15-, and 20-mg vortioxetine groups, respectively, and 11.3% (50/442) for the duloxetine group. Zero to 0.4% of patients in the vortioxetine groups and <0.1% in the placebo group reported suicidal behavior. No suicidal behavior was reported for the duloxetine group. One aborted suicide attempt in the placebo group, one preparatory act in the 5-mg vortioxetine group, and two nonfatal suicide attempts in the 10-mg vortioxetine group were reported during the treatment period.

FIGURE 2. Suicidal ideation or behavior based on C-SSRS at baseline and during entire treatment period in seven short-term MDD studies. For studies included, see Table 1. Percentages of patients with any suicidal ideation or behavior based on the C-SSRS score of 1–9 are shown. Baseline period includes events that occurred between screening and baseline visits. Patients were counted once using the most severe C-SSRS event. Patients could have also reported nonsuicidal self-injurious behavior; these nonsuicidal events were not included in determining the most severe event. DLX, duloxetine; MDD, major depressive disorder; n, total number of patients assessed with C-SSRS; PBO, placebo; VOR, vortioxetine.
C-SSRS data were evaluated for patients with MDD who completed the short-term lead-in studies and entered into the long-term extension trials (long-term pool; N = 1956) (Table 5). During the treatment period, 90% of patients (n = 1748) taking vortioxetine reported no suicidal ideation or behavior. Any suicidal ideation or behavior was reported by 41.6% of patients (n = 813) in their lifetime assessment (all prior history) and by 10.0% of patients (n = 194) during the treatment period. Suicidal behavior was reported by 13.3% (n = 260) of patients in their lifetime histories, whereas 0.2% (n = 4) reported suicidal behavior during the treatment period, which included one preparatory act, one aborted attempt, and two nonfatal suicide attempts. No completed suicides occurred during the long-term trials (Table 5).
TABLE 5. Suicidal ideation and behavior based on C-SSRS using all prior history and treatment period assessments in the MDD long-term pool*

Notes: C-SSRS, Columbia-Suicide Severity Rating Scale; MDD, major depressive disorder.
Values are number of patients (%). For studies included, see Table 2.
* Patients were counted once using the most severe event. Patients could have also reported nonsuicidal self-injurious behavior, and thesenonsuicidal events were not included in determining the most severe event. All prior history includes events that occurred before administration of the study drug in the initial double-blind study and at screening and baseline visits.
Shifts in C-SSRS categories
Categorically, most patients in both the short-term and long-term pools shifted toward a positive direction (i.e., a decrease in suicidal ideation or suicidal behavior). Among patients in the short-term pool with no suicidal ideation or behavior at baseline, >90% of MDD patients in all treatment groups remained in this category during the treatment period (Table 6). Of the patients with no suicidal ideation or behavior at baseline, 9.7% (99/1019) in the placebo group, 5.7% (22/384) in the duloxetine group, and 6.8% (32/471), 6.7% (26/387), 6.3% (25/395), and 6.8% (26/385) in the 5-, 10-, 15-, and 20-mg vortioxetine groups, respectively, shifted to suicidal ideation (Table 6). Of the patients who reported suicidal ideation at baseline, 40.9% (72/176) in the placebo group, 51.7% (30/58) in the duloxetine group, and 29.8% (34/114), 42.1% (24/57), 36.7% (18/49), and 33.3% (20/60) in the 5-, 10-, 15-, and 20-mg vortioxetine groups, respectively, shifted to the improved category of no suicidal ideation or behavior. A shift from no suicidal ideation or behavior at baseline to suicidal behavior was observed in one patient (0.2%) in the 5-mg vortioxetine group (1/471) and one patient (0.3%) in the 10-mg vortioxetine group (1/387).
TABLE 6. Shifts in C-SSRS categories from baseline to worst postbaseline assessment during the treatment period in the MDD short-term pool*

Notes: C-SSRS, Columbia-Suicide Severity Rating Scale; DLX, duloxetine; MDD, major depressive disorder; n, number of patients in each category at baseline, and denominator for percentage; VOR, vortioxetine.
Baseline period includes events that occurred between screening and baseline visits. Patients were counted once using the most severe event. Patients could have also reported nonsuicidal self-injurious behavior; these nonsuicidal events were not included in determining the most severe event.
Shift analyses of the long-term pool (2.5–20 mg of vortioxetine) (Table 7) revealed that of the 1134 patients who reported no suicidal ideation or behavior in their lifetime assessment (all prior history), 97.8% (n = 1109) remained in this category during the study, whereas 2.1% (n = 24) of patients shifted to a worse category of suicidal ideation and <0.1% of patients (n = 1) to suicidal behavior. Of 550 patients with a lifetime history of suicidal ideation, 78.5% (n = 432) shifted to an improved category of no suicidal ideation or behavior, and 0.2% (n = 1) worsened and shifted to the suicidal behavior category. Of the 258 patients who reported suicidal behavior in their lifetime histories, 80.2% (n = 207) improved to no suicidal ideation or behavior, and 19% (n = 49) improved to suicidal ideation. Two patients (0.8%) remained in the suicidal behavior category.
TABLE 7. Shifts in C-SSRS categories from all prior history to worst postbaseline assessment during the treatment period in the MDD long-term pool*

Notes: C-SSRS, Columbia Suicide Severity Rating Scale; MDD, major depressive disorder; VOR, vortioxetine.
All prior history includes events that occurred before administration of study drug in the open-label period. Patients were counted once using the most severe event. Patients could have also reported nonsuicidal self-injurious behavior; these nonsuicidal events were not included in determining the most severe event.
Suicide-related events
Suicide-related events were further investigated using reported TEAEs in addition to C-SSRS assessments. No completed suicides occurred during any of the studies. The incidence of suicide-related events was low and similar for all treatment groups, with no dose-related increases observed among the individual vortioxetine groups in the short-term pool (N = 4990) (Table 8). One percent or less of the patients treated with vortioxetine (0.2% [2/1013], 1% [7/699], 0.7% [3/449], and 0.7% [3/455] for 5, 10, 15, and 20 mg, respectively), 0.4% (7/1621) treated with placebo, and 0.7% (5/753) treated with duloxetine showed suicidal ideation and behavior (Table 8).
TABLE 8. Overview of suicide-related event (TEAEs by Preferred Term) in the MDD short-term pool*

Note: Values are number of patients (%).
MDD, major depressive disorder; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; SMQ, Standardized MedDRA Queries suicide/self-injury; TEAE, treatment-emergent adverse event.
* For studies included, see Table 1.
The incidence of suicide-related events was low in the long-term pool (N = 1956). A total of 12 patients (0.6%) had suicidal ideation and behavior. Of these, nine patients (0.5%) had suicidal ideation, two patients (<0.1%) attempted suicide, one patient (<0.1%) had self-injurious ideation, and one patient had self-injurious behavior (<0.1%) (Table 9).
TABLE 9. Suicide/self-injury SMQ overview of suicide-related events (TEAE by Preferred Term) in the MDD long-term pool*
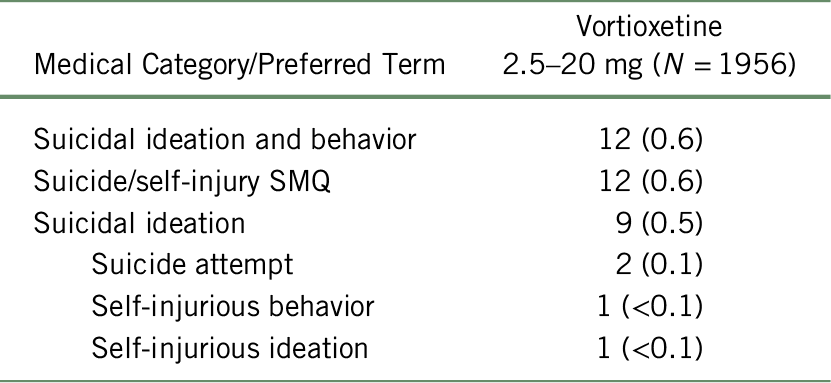
Notes: MDD, major depressive disorder; MedDRA, Medical Dictionary for Regulatory Activities; SMQ, Standardized MedDRA Queries suicide/self-injury; TEAE, treatment-emergent adverse event.
Values are n (%). Patients with one or more adverse events within a level of the Medical Category, Medical Query, or MedDRA Preferred Term are counted only once in that level.
* For studies included, see Table 2. Adverse events occurring on or after the first dose and within 30 days after dosing are included. MedDRA Preferred Terms are sorted in descending order based on incidence of LuAA21004 Total.
Discussion
The safety and tolerability of vortioxetine have previously been evaluated in a comprehensive global clinical development program, which included an analysis of pooled data using the vortioxetine clinical trial database.Reference Baldwin, Chrones and Florea31 Suicidal ideation and behavior were assessed using the C-SSRS in eight 8-week placebo-controlled studies in MDD and in three of the open-label long-term studies. This study was an overall assessment of the safety and tolerability of vortioxetine, and it included additional safety and tolerability data from four published short-term, placebo-controlled studies in GAD. Differences between vortioxetine treatment and placebo groups in this study were not clinically relevant.
These post hoc analyses evaluated pooled data from 10 short-term (6 or 8 weeks), randomized, placebo-controlled trials (N = 4990 patients) and 3 open-label, long-term extension trials (N = 1956 patients) and indicate that vortioxetine was not associated with an increased risk of suicidal ideation and behavior in adult patients with MDD. The incidence of suicidal ideation and behavior as a result of vortioxetine administration was similar to that of placebo, and no dose-related increases were observed among the different vortioxetine treatment groups (5–20 mg). Long-term treatment (52 weeks) with vortioxetine in adults with MDD did not raise the risk of suicidal ideation and behavior. There were no completed suicides in any treatment group.
In the short-term pool, suicide-related events (including the preferred terms suicidal ideation, intentional overdose, suicide attempt, intentional self-injury, and self-injurious behavior) were low and similar among treatment groups. Of the 2616 patients treated with vortioxetine, 19 (0.6%) reported any suicidal ideation or behavior compared with 7 (0.4%) of the 1621 patients treated with placebo and 5 (0.7%) of the 753 treated with duloxetine. Similarly low incidences of suicidal ideation and behavior based on TEAEs were reported in the long-term pool (eight patients [0.7%]). These data agree with previously reported suicide-related events data for vortioxetine, which did not show clinically relevant differences for vortioxetine versus placebo, nor did the data show dose effects.Reference Baldwin, Chrones and Florea31
Similar results were reported in systematic reviews and pooled analyses of randomized, controlled studies for other antidepressants in patients with MDD and GAD; none of the antidepressants investigated increased the risk of suicidal ideation and behavior based on standard depression symptom rating scales such as HAM-D or MADRS.Reference Acharya, Rosen and Polzer15, Reference Pedersen17, Reference Kasper, Montgomery and Moller22 Additional post hoc studies evaluated suicidal ideation and behavior based on C-SSRS and suicide-related TEAEs. For example, post hoc analyses of data for levomilnacipran extended release (ER), a serotonin and norepinephrine reuptake inhibitor, found no differences between placebo and short-term levomilnacipran ER use and no increase in suicidal ideation and behavior for longer courses of continued treatment. Pooled data for clinical trials of GAD and MDD showed that the incidences of suicidal ideation and behavior based on C-SSRS were similar or lower than placebo for vilazodone, a combined reuptake inhibitor and a 5-HT1A receptor partial agonist approved for MDD in 2011.Reference Thase, Edwards and Durgam46 The aforementioned studies agree with the meta-analyses of 207 randomized controlled trials of antidepressants and suicide-related events evaluated by the FDA.Reference Hammad, Laughren and Racoosin14 Taken together, independent FDA analyses and data from various clinical trials suggest the safety of antidepressants and low risk of suicidal behavior in MDD patients taking antidepressants.
Measured using the C-SSRS, suicidal ideation or behavior was low, with no differences between the vortioxetine groups and placebo group in the short-term pool during the treatment period. Suicidal ideation or behavior was 17% (204/1199) in the placebo group, 15.4% (296/1924) in the 5- to 20-mg vortioxetine groups combined (19.3% [113/586], 13.5% [60/446], 12.6% [56/445], and 15% [67/447] for 5, 10, 15, and 20 mg of vortioxetine, respectively), and 11.3% (50/442) in the duloxetine group (Table 4).
C-SSRS shift data showed that categorically, most patients either did not shift or shifted to a category with an improved postbaseline assessment of suicidal ideation or behavior incidence. C-SSRS shifts from no suicidal ideation or behavior at baseline to suicidal ideation during treatment were low and similar across treatment groups: 9.7% (99/1019) for placebo, 6.7% (109/1638) for all patients in the 5- to 20-mg vortioxetine groups combined, and 5.7% (22/384) for patients in the duloxetine treatment group. Two (0.12%) of the 1638 patients in the 5- to 20-mg combined vortioxetine groups shifted from suicidal ideation or behavior to suicidal behavior. A recent publication featuring the safety and tolerability of vortioxetine reported similar data.Reference Baldwin, Chrones and Florea31 In Baldwin et al.,Reference Baldwin, Chrones and Florea31 C-SSRS shift analysis comparing lifetime events with any postbaseline event during the study showed a shift from the category “no suicidal ideation or behavior” to the category “suicidal ideation” for 4.1% (34/836) in the placebo group and 2.7% (39/1464) in the vortioxetine group (5–20 mg), whereas 0.1% (2/1464) in the vortioxetine group (5–20 mg) shifted to suicidal behavior. No clinically relevant differences were observed on newly emergent suicidal ideation or behavior between placebo and vortioxetine; suicidal ideation was reported for 16.1% (224/1393) in the placebo group and 14.6% (338/2322) in the vortioxetine groups (5–20 mg) during treatment.Reference Baldwin, Chrones and Florea31
In the long-term pool evaluating flexible doses of vortioxetine, 9.8% (n = 190) and 0.2% (n = 4) of 1942 patients reported suicidal ideation and suicidal behavior, respectively, based on C-SSRS during the 52-week treatment period. Over time, most patients shifted toward less suicidal ideation or behavior. Of the patients who reported prior histories of suicidal ideation or behavior, 78.5% (432/550) and 80.2% (207/258), respectively, shifted to neither suicidal ideation nor behavior during the study. Two (0.1%) of 1942 patients shifted to a worse category of suicidal behavior. Previously reported data from each long-term study individually show no, or comparably low, rates of measured suicidal ideation or behavior, regardless of vortioxetine dosage.Reference Fillipov and Christens20, Reference Alam, Jacobsen and Chen42, Reference Jacobsen, Harper and Chrones43
Data from this study suggest that short-term and long-term treatment with vortioxetine is not associated with increased risk of suicidal ideation or behavior in patients with MDD.
Study Limitations
Not all studies used the C-SSRS data for assessment of suicidal ideation or behavior. Therefore, slight underestimations or overestimations of the categorical shifts might exist. In addition, an accurate assessment of antidepressant-associated suicidal behavior within clinical trials has traditionally been challenging because of exclusion criteria preventing high-risk suicide patients from participating in the trials, and these post hoc analyses included clinical trials that had adopted the same criteria.Reference Grunebaum, Ellis and Duan47
Conclusion
Treatment with vortioxetine was not associated with an increased risk of suicidal ideation or behavior in adults with MDD. Overall, these analyses are consistent with previously published clinical trial data investigating the risk of antidepressants on suicidal ideation or behavior.
Acknowledgments
Assistance with manuscript preparation was provided by Andrea McReynolds, PhD, and Martina Schwarzkopf, PhD, of inVentiv Medical Communications, LLC, a Syneos Health™ group company, and supported by Takeda Pharmaceutical Company, Ltd., and H. Lundbeck A/S. All authors assume full responsibility for the scientific content of the manuscript.
Funding statement
The study was funded by Takeda Pharmaceutical Company, Ltd., and H. Lundbeck A/S.
Disclosures
John Affinito is an employee of Takeda Development Center Americas, Inc. Atul R. Mahableshwarkar, George Nomikos, Judith Xu, and Paula L. Jacobsen were employees of Takeda Development Center Americas, Inc., at the time of the study. Elin Heldbo Reines is an employee of H. Lundbeck A/S.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S109285291900097X















