Introduction
Functional movement disorder (FMD), the motor-dominant subtype of functional neurological disorder (FND), is a complex neuropsychiatric syndrome characterized by positive clinical findings.Reference Espay, Aybek and Carson 1 Although FMD is diagnosed based on the dominant movement disorder, there are often additional non-motor features present such as pain, fatigue, or other functional symptoms. Debate persists about “lumping” or “splitting” FMD into phenotypic subgroups.Reference Lidstone, Costa-Parke, Robinson, Ercoli and Stone 2 , Reference Tinazzi, Geroin and Marcuzzo 3 Recent studies investigating patient differences between FMDs have not found clinically relevant subgroups, and up to half of patients may experience changes in the movement disorder during their disease course.Reference Tinazzi, Geroin and Marcuzzo 3 -Reference Ercoli, Tinazzi and Geroin 7 A recent study identified 2 distinct subtypes of FMD (tremor/parkinsonism and weakness) when considering patients with multiple motor presentations; however, psychological factors were not included.Reference Mostile, Geroin and Erro 8
Given that FMD is a complex neuropsychiatric illness, it is probable that phenotypic differences do exist when broadening the view to include mental health factors alongside motor symptoms. When seen through a transdisciplinary lens, many patients with FMD manifest psychological traits, behavioral patterns, personality styles, and other factors that are relevant to the development and maintenance of FMD.Reference McKee, Glass and Adams 9 -Reference Maggio, Kyle, Stephen and Perez 11 These “FMD-relevant” factors span physical domains (eg, signs of autonomic hyperarousal and a dysregulated stress response) and psychological domains (eg, perfectionism, low self-agency, and avoidance patterns).Reference Paredes-Echeverri, Maggio, Bègue, Pick, Nicholson and Perez 12 , Reference Keynejad, Frodl, Kanaan, Pariante, Reuber and Nicholson 13 Importantly, these “ingredients” are not inherently pathological, nor are they routinely assessed on psychiatric evaluation, but nevertheless contribute to the expression of FMD acting as predisposing or perpetuating factors, either alone or in combination.Reference Maggio, Kyle, Stephen and Perez 11 , Reference Erten, Yenilmez, Fistikci and Saatcioglu 14 -Reference Demartini, Petrochilos, Ricciardi, Price, Edwards and Joyce 16 These features may also intersect with the personal history (eg, adverse life events) which are recognized risk factors for developing FMD.Reference Ludwig, Pasman and Nicholson 17 , Reference Roelofs, Keijsers, Hoogduin, Näring and Moene 18 Although psychiatry has long understood the importance of many of these FMD-relevant factors—and in particular their relevance for functional symptom maintenance—substantial gaps remain in their integration with neurological considerations, especially FMD phenotype recognition and treatment approaches. Integrated FMD clinics, in which co-assessments by neurology, psychiatry, and allied health occur, are uniquely poised to explore these concepts in a data-driven way.
The primary objective of this retrospective chart review was to explore potential novel, neuropsychiatric FMD phenotypes by combining neurological, psychiatric, and FMD-relevant factor assessments, to generate testable hypotheses that can direct future research. In our clinical experience using an integrative assessment process, we observed possible phenotypes such as patients with episodic hyperkinetic movements having evidence of hyperarousal, and weakness tending to be a constant symptom that co-occurred with activity avoidance. We hypothesized that when incorporating these FMD-relevant factors into an integrative assessment process, phenotypes would emerge. Secondary aims were to use the detailed neuropsychiatric database to further characterize and report concepts previously identified in the literature, including triggering events, changing FMD presentation over time, defining an FND syndrome of non-motor features, and the contribution of adverse life experiences to certain phenotypic presentations.
Methods
Study design
A retrospective chart and video review was performed of all consecutive patients with a diagnosis of FMD evaluated at the Toronto Rehabilitation Institute Integrated Movement Disorders Program (IMDP) in Toronto, Canada between July 2019 and December 2021.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the University Health Network Research Ethics Board (REB 21-6172, approved February 11, 2022).
Eligibility criteria and clinical assessment
FMD was diagnosed based on clinical interviews and examination by an experienced movement disorders specialist based on positive clinical signs. 19 , Reference Gupta and Lang 20 Initial assessment occurred in person at the Toronto Western Hospital Movement Disorders Clinic, where the diagnosis of FMD was confirmed and delivered, and referral generated to the IMDP, a subspecialist FMD clinic providing rehabilitation. At this visit, patients were assessed in person or virtually by a movement disorders neurologist (S.C.L.) and neuropsychiatrist (L.M.) in an integrated fashion.Reference Lidstone, MacGillivray and Lang 21 The neuropsychiatric clinical assessment was open-ended and qualitative in nature, prioritizing a review of personal history and identification of FMD-relevant factors (Table 1).
Table 1. Description of FMD-Relevant Factors

Abbreviation: FMD, functional movement disorder.
Inclusion criteria included: (1) clinical diagnosis of FMD, agreed upon by 2 movement disorders neurologists; (2) at least 1 assessment in the IMDP; (3) documentation from 2 separate clinic visits at 2 time points, that is, movement disorders clinic consultation and IMDP consultation; and (4) age ≥ 18 years.
Data extraction
Records reviewed included the initial movement disorders consultation with video examination, IMDP assessment with video, and intake questionnaire completed by the patient prior to the IMDP visit. Demographic and clinical factors were extracted by a single reviewer (G.S.G.) and verified by 2 additional reviewers (S.C.L. and L.M.) on a case-by-case basis. Data extracted from the initial movement disorders consultation included demographic variables and movement disorders phenotype. Data extracted from the IMDP consultation included: demographic variables, functional movement symptoms, psychiatric diagnoses using DSM-5 criteria, non-motor symptoms (including pain, fatigue, cognitive fog, functional seizures, and other FND symptoms), FMD-relevant factors, medical profile, family history, previous investigations/treatment, and trauma/abuse history. Pain was reported by patients and could include any origin (neuropathic and nociceptive), severity (not typically quantified), and location of pain. The description of cognitive fog was not systematically evaluated, but likely included a variety of cognitive experiences such as forgetfulness, poor concentration, and excessive cognitive effort.Reference McWhirter, Smyth, Hoeritzauer, Couturier, Stone and Carson 22 Functional seizures were defined as episodes resembling epilepsy or syncope, but not caused by abnormal cortical electrical activity.Reference Hallett, Aybek, Dworetzky, McWhirter, Staab and Stone 23 Data extracted from the patient intake forms included: FMD triggering events, the top 3 most concerning symptoms, a review of symptoms checklist, and functional capacity. Adverse life events history was assessed based on self-reported data disclosed on the intake form, and further explored during the IMDP assessment. Further details regarding the reporting of trauma and abuse are included in the Supplemental Methods.
Abnormal movements were classified into the following categories based on common FMD presentations: gait disorders, tremor, appendicular jerks/myoclonus, axial jerks/myoclonus, fixed dystonia, weakness, parkinsonism (appearance of increased tone, slowness, with or without gait disorder and tremor), facial movements, and tics. If multiple movement symptoms were present, each was noted. Videos of the examinations were used to confirm movement disorder presentations. Motor symptoms were further classified as episodic or constant, based on the combined features of history and examination. Episodic motor symptoms were characterized as occurring either intermittently or in discrete “attacks,” separated by definable periods without motor symptoms, recognized and reported by the patient. The examination could be normal unless motor symptoms occurred during the assessment, either spontaneously or triggered. By contrast, constant movement symptoms were reported by the patient to be continuously present, and consistently visible throughout the entire assessment to the examiner. Positive signs of distractibility or inconsistency were not sufficient to classify a movement symptom as episodic, and similarly, did not preclude a symptom from being considered constant. Further characterization of episodic vs. constant motor symptoms is described in the Supplemental Methods.
We define FMD-relevant factors as recurrent, observable behavioral patterns identified by clinicians with subspecialist experience in evaluating and treating patients with FMD. These factors are drawn from the psychiatric literature and clinical experience. Some have been previously associated with FND, whereas others are well-recognized phenomena in psychiatry without being specifically linked to FND (eg, coping style and personality traits).Reference McKee, Glass and Adams 9 -Reference Maggio, Kyle, Stephen and Perez 11 Although readily identifiable, most of these factors lack standardized questionnaires or scales to evaluate. If present, these factors were explicitly documented during the history and examination. See Table 1 for a detailed list and description of the included FMD-relevant factors.
Statistical analyses
All statistics were performed on SAS 9.4 (SAS Institute, Cary, North Carolina, USA) for Windows. The sample size was based on the number of eligible patients whose charts were available between July 2019 and December 2021. Skewness was assessed in continuous variables, and sparsity and homogeneity were assessed in categorical variables. Univariate group differences were analyzed by Fischer’s exact tests for categorical variables, independent t-tests for continuous normally distributed variables, and Mann–Whitney U tests for ordinal variables such as measures utilizing Likert scales. Exploratory hierarchical cluster analyses using a leader algorithm for movement disorder presentations and psychological traits were performed to generate potential directional hypotheses for multivariable logistic regressions. Variables with sparse cell counts in the variable-outcome contingency table were excluded from the models. Cluster analyses included datasets with movement disorder phenotypes alone, and movement disorder phenotypes combined with non-motor symptoms, psychiatric diagnoses, and FMD-relevant features. Logistic regression models were built to analyze the relationships between variables of interest (OR, 95% confidence interval [CI]). Variable selection for final exploratory models was performed using backward elimination methods to remove variables with small estimate values (<.001), homogeneous and sparse variables. Collinearity was also assessed, and models were compared with the variables coded separately and as combined variables to determine model fit. Values are expressed as mean (standard deviation [SD]) or median and interquartile range (IQR; Q1, Q3), as appropriate, for continuous variables, and as counts and percentages for categorical variables. A P-value <.05 was considered statistically significant.
Results
Charts were reviewed for all consecutive 159 patients assessed with a referring diagnosis of FMD. One patient was excluded due to a revision of diagnosis to Parkinson’s disease without evidence of FMD. In total, 158 patients were included in the final analysis. Videos were available and reviewed from the movement disorders clinic for 73 patients (46%), and from the IMDP for 64 patients (40%). When a video was not available, a detailed movement disorder description from the chart was sufficient for classification. The mean time between visits was 8.2 (SD 8.3) months.
Patient characteristics
General patient demographics and FMD characteristics are presented in Tables 2-4. Most patients were female (71%). The mean age at the time of assessment was 47.3 (SD 14.6) years. The mean age of onset of FMD was 39.9 (SD 15.3) years, with a delay in diagnosis of median 7.5 (IQR 3, 21.5) years. FMD triggering events were identified in 86% of the patients (Table 3).
Table 2. Patient Demographics and Self-Reported Variables
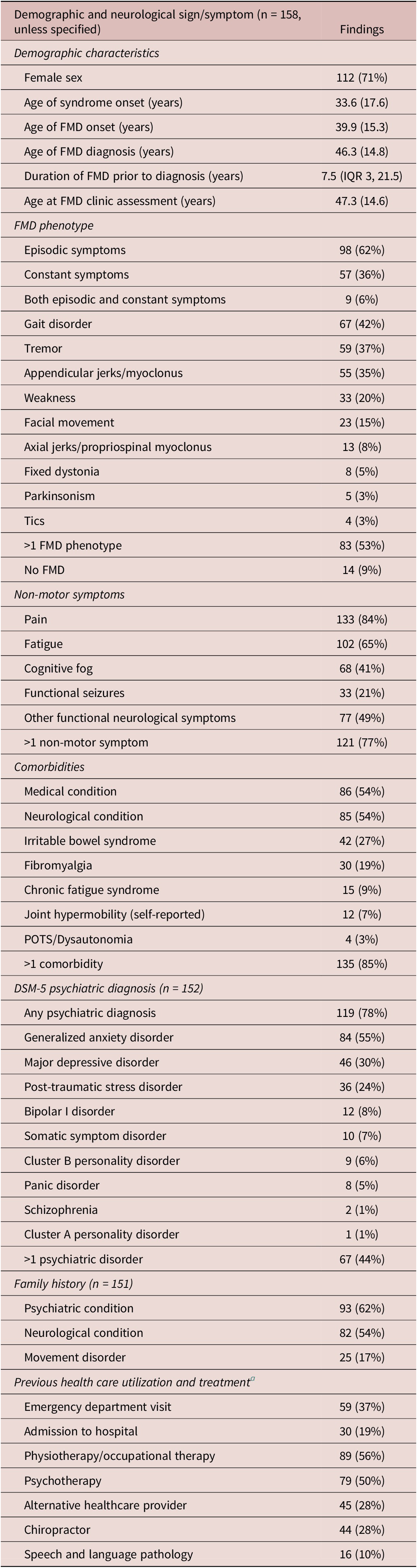
Abbreviations: FMD, functional movement disorder; POTS, postural orthostatic tachycardia syndrome.
a Self-reported data.
Table 3. Self-Reported Variables
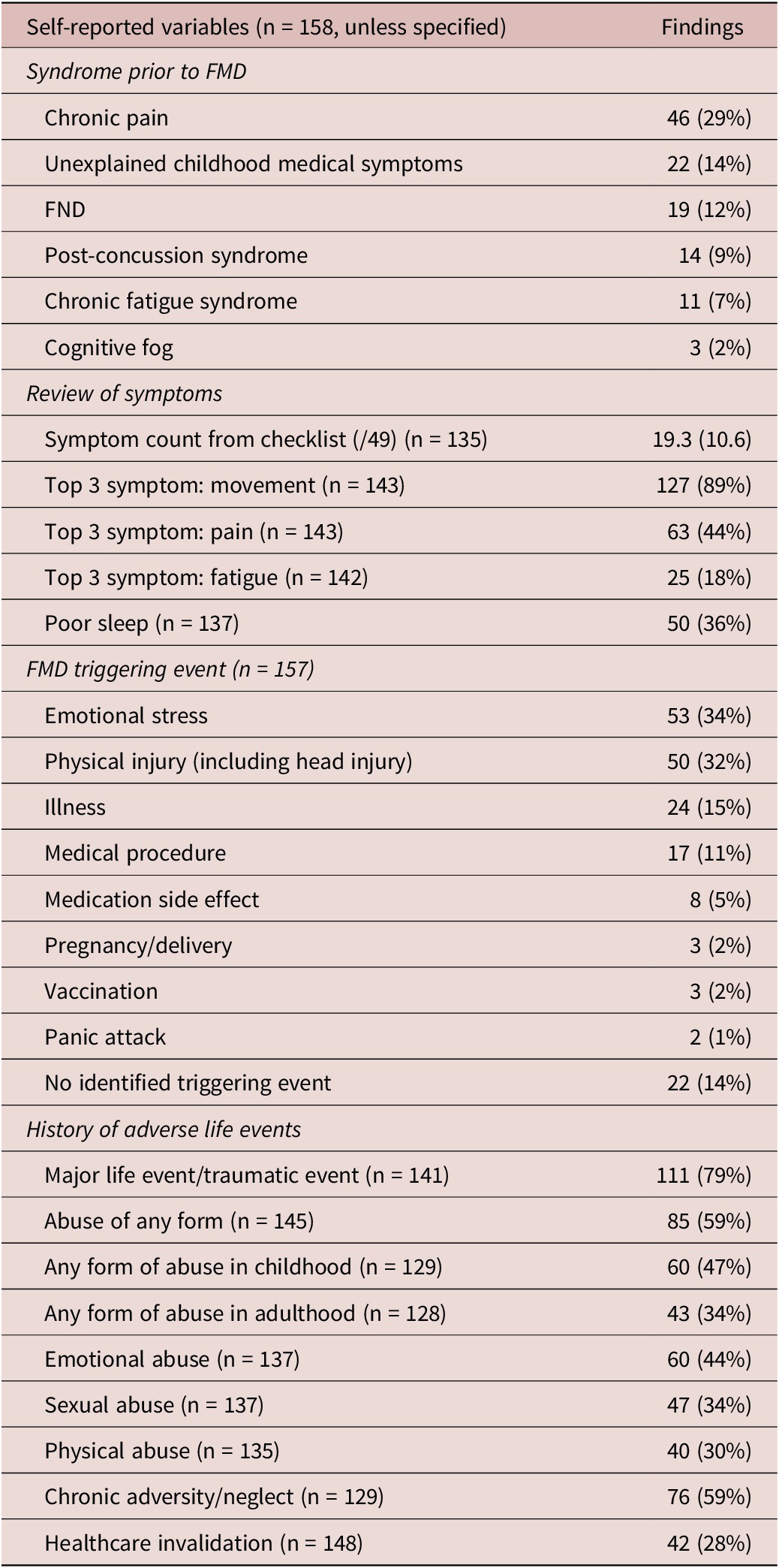
Abbreviations: FMD, functional movement disorder; FND, functional neurological disorder.
Table 4. FMD-Relevant Characteristics
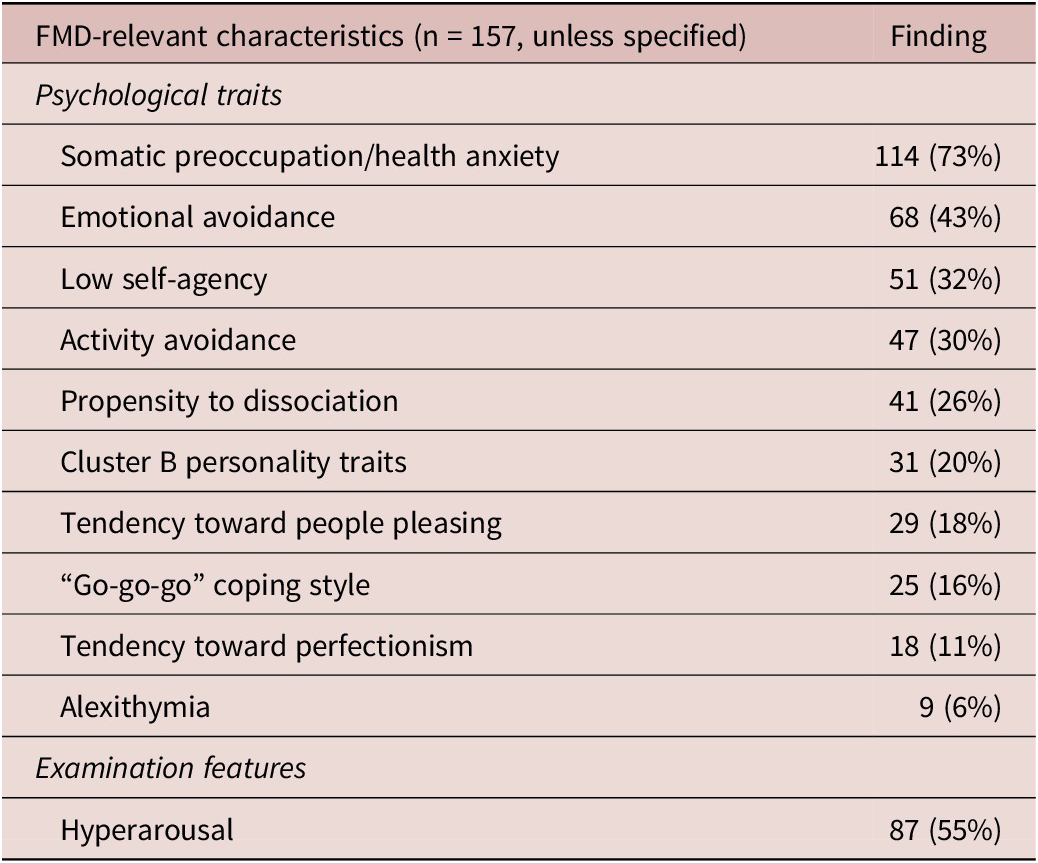
Abbreviation: FMD, functional movement disorder.
Movement disorder characteristics
Episodic movement symptoms (62%) were more common than constant symptoms (36%). Gait disorders (42%), tremors (37%), appendicular jerks/myoclonus (35%), and weakness (20%) were most frequent, and 53% of patients had greater than 1 movement disorder symptom. Notably, 94% of patients with appendicular jerks/myoclonus demonstrated mixed symptoms with a combination of multiple hyperkinetic movements in addition to jerks, including dystonia (59%), gait disorder (46%), tremor (39%) and facial movements (24%) (Supplemental Figure 1). Thirteen patients (24%) in this group had co-existing functional weakness. Fifty-eight patients (42%) had a change in their movement disorder presentation between assessments, which was not influenced by symptom duration (P = .541) or the time that lapsed between appointments (P = .575) (Supplemental Table 1). Symptoms typically remained as episodic (79% no change) or constant (72%). Fourteen patients (9%) had resolution of their movement disorder between assessments; however, all had persistent FND symptoms.
Non-motor symptoms
Non-motor symptoms were common, including pain (84%), fatigue (65%), other FND symptoms (49%), cognitive fog (41%), and functional seizures (21%) (Table 3). The mean self-reported symptom count out of 49 possible symptoms was 19.3 (10.6). Patients reported other functional symptoms predating the onset of FMD including chronic pain (29%), unexplained childhood medical symptoms (14%), and FND without movement symptoms (12%). The mean age of this non-motor functional symptom onset was 33.6 (17.6) years, 6.3 years earlier than the onset of movement symptoms.
Psychiatric profiles
Most patients (78%) had a DSM-5 psychiatric diagnosis either previously received and confirmed by the psychiatrist during a consultation, or diagnosed at the time of consultation. These included generalized anxiety disorder (GAD; 55%), major depressive disorder (30%), and post-traumatic stress disorder (PTSD; 24%). One third (32%) of patients had a change of DSM-5 diagnosis at the time of subspecialist FND assessment, either through a new diagnosis being made, or occasionally when a historical diagnosis was revised due to lack of supportive evidence (Supplemental Table 2). The most common newly made psychiatric diagnoses included GAD (n = 24) and PTSD (n = 12).
FMD-relevant factors
Frequently identified FMD-relevant factors are summarized in Table 4. Somatic preoccupation/health anxiety (73%), emotional avoidance (42%), low self-agency (32%), and activity avoidance (30%) were most common. Signs of hyperarousal were observed in 55% of patients.
Exploratory neuropsychiatric phenotype analysis
Using cluster analysis, we examined the relationships between movement disorder presentations and then explored if these were associated with distinct psychiatric diagnoses or FMD-relevant factors.
The exploratory cluster analysis revealed 3 distinct movement disorder clusters, visually presented in Supplemental Figure 2a: (1) episodic motor symptoms and tremor; (2) episodic motor symptoms and appendicular jerks/myoclonus; and (3) constant motor symptoms and gait disorder. Clusters combining movement disorder presentations with non-motor and FMD-relevant factors included (Supplemental Figure 2b): (1) episodic symptoms, tremor, hyperarousal, and cognitive fog; (2) episodic symptoms, appendicular jerks/myoclonus, and hyperarousal; (3) constant symptoms, gait disorder, activity avoidance, and low self-agency; and (4) constant symptoms, gait disorder, weakness, and emotional avoidance. Health anxiety/somatic preoccupation, pain, and fatigue were ubiquitously present in every cluster and so were combined as a single factor in the analysis.
Movement disorder phenotypes
Exploratory logistic regression models were built to study the possible association of episodic and constant symptoms with movement disorder presentations (Table 5). Phenotypes associated with episodic symptoms included: appendicular jerks/myoclonus (OR 11.32, 95% CI 4.11-31.14), tremor (OR 5.41, 95% CI 2.14-13.71), and axial jerks (OR 20.86, 95% CI 2.33-187.09). There was a negative association with gait (OR 0.35, 95% CI 0.15-0.84). In contrast, phenotypes associated with constant symptoms included: gait disorders (OR 7.32, 95% CI 3.07-17.48), weakness (OR 4.88, 95% CI 1.76-13.53), and fixed dystonia (OR 12.36, 95% CI 1.89-80.94). There was a negative association with appendicular jerks/myoclonus (OR 0.32, 95% CI 0.12-0.78).
Table 5. Logistic Regression Models Examining Relationships between Functional Movement Disorder Phenotype and Episodic vs. Constant Symptoms
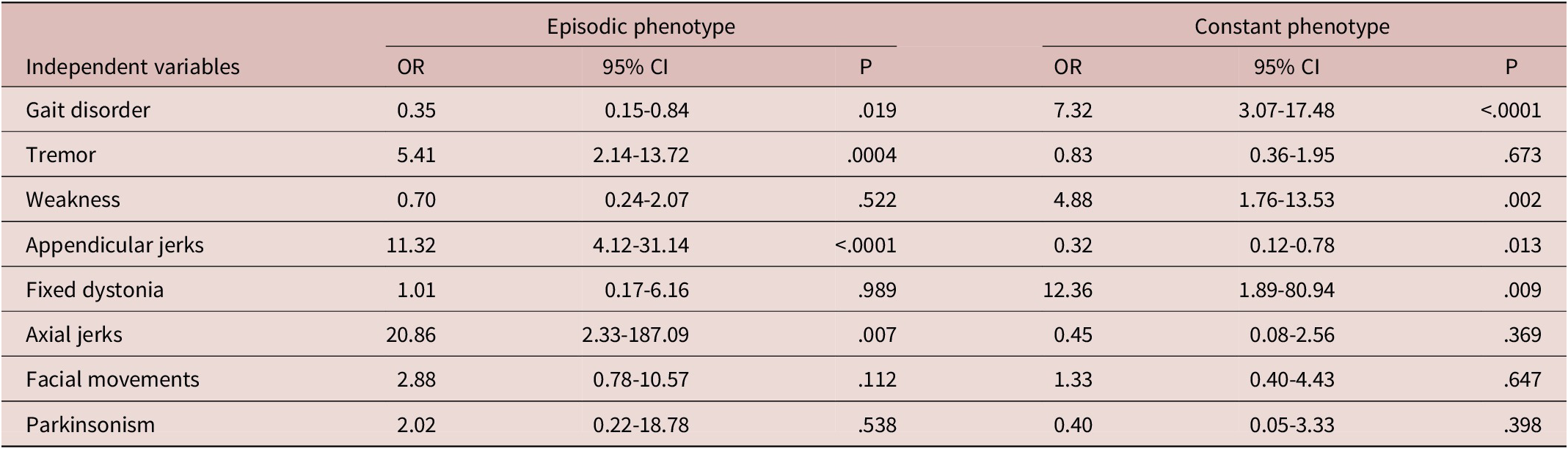
Abbreviations: CI, confidence interval; OR, odds ratio.
Combined movement and psychological phenotypes
Exploratory logistic regression models were built to investigate the association of movement disorder phenotype with psychiatric diagnosis and FMD-relevant psychological factors (Supplemental Table 3). Movement disorder presentations were not associated with DSM-5 diagnoses, except for a negative association between depression and gait disorder (OR 0.37, 95% CI 0.15-0.91). Low self-agency was associated with weakness (OR 2.53, 95% CI 1.06-6.02). Hyperarousal was associated with appendicular jerks/myoclonus (OR 2.14, 95% CI 1.03-4.46), but not tremor (OR 1.51, 95% CI 0.73-3.11). Neither activity avoidance nor emotional avoidance was associated with a constant movement disorder phenotype (OR 1.15, 95% CI 0.54-2.44; OR 0.73, 95% CI 0.36-1.47, respectively).
Psychiatric and FMD-relevant phenotypes
Exploratory logistic regression models were built to investigate associations between psychiatric and FMD-relevant psychological factors (Supplemental Table 4). PTSD and major depressive disorder were associated with cluster B personality traits (OR 4.74, 95% CI 1.84-12.22; OR 2.61, 95% CI 1.01-6.45, respectively). Activity avoidance was associated with low self-agency (OR 2.40, 95% CI 1.14-5.04), but emotional avoidance was not (OR 1.51, 95% CI 0.65-3.07) (Supplemental Table 3). Hyperarousal was associated with GAD (Fischer’s exact 2-tailed test P < .0001), but not somatic preoccupation/health anxiety (Fischer’s exact 2-tailed test P = .136). Higher symptom count had an association with low self-agency and propensity to dissociate (OR 3.98, 95% CI 1.97-8.06; OR 3.05, 95% CI 1.48-6.27, respectively). Symptom count was not associated with GAD (OR 1.01, 95% CI 0.51-1.98) or somatic preoccupation/health anxiety (OR 1.84, 95% CI 0.66-5.15) (Supplemental Table 3).
Adverse life events sub-analysis
A history of a life-threatening traumatic experience (79%), any abuse (59%), and chronic adversity/neglect (59%) were commonly reported (Table 3). Patients with a history of abuse reported it occurring both in childhood and adulthood (P < .001). Similar rates of abuse were present in men and women, except for history of sexual abuse, which was more frequent in women than men (47% women vs. 7% men, P < 0.0001). Adverse life events were not associated with any specific movement disorder phenotype (Supplemental Table 5). History of sexual abuse had a negative association with a constant phenotype (OR 0.19, 95% CI 0.04-0.85), but was not associated with episodic phenotype (OR 0.62, 95% CI 0.16-2.46). History of any abuse, sexual abuse, and chronic adversity/neglect were associated with hyperarousal (OR 3.15, 95% CI 1.36-7.30; OR 2.24, 95% CI 1.02-4.91; and OR 2.36, 95% CI 1.11-5.00, respectively).
Discussion
This retrospective chart review describes a sample of 158 consecutive patients evaluated using an FMD subspecialist integrated assessment. This transdisciplinary approach confirmed and expanded upon previously described clinical features of FMD, and also provided novel observations spanning the neurological-psychiatric interface: (1) distinct FMD phenotypes emerge not when stratifying by movement disorder phenomenology, but instead when stratifying by episodic vs. constant motor symptoms; (2) episodic FMD is characterized by hyperkinetic symptoms including tremor and jerks, and is associated with anxiety, hyperarousal, and history of trauma; (3) constant FMD is characterized by gait disorders, weakness, or fixed dystonia, and is associated with activity avoidance and low self-agency; and (4) the movement disorder is part of a broader FMD syndrome with prominent non-motor symptoms. Although clearly only hypothesis-generating at this stage, these results suggest that clinically meaningful signals emerge when considering more domains of neuropsychiatric function beyond the movement disorder alone, which can inform how we understand the development, maintenance, and treatment of FMD. The clinical heterogeneity of FMD, however, reinforces that overlap can exist between these 2 groups, for example, a patient presenting with a combined constant gait disorder and episodic tremor.
These results suggest that FMD can be broadly grouped into episodic and constant movement phenotypes and that these 2 groups are associated with distinct neuropsychiatric/psychological features (Figure 1). Functional symptoms fluctuate by their very nature, but the presence of identifiable periods without motor symptoms that is recognized by the patient separates the episodic from the constant group. Episodic FMD is characterized by hyperkinetic movements experienced as discrete attacks, or intermittently in specific situations, indicating triggering factors (known or unknown to the patient) and sensitivity to environmental cues.Reference Geroin, Stone, Camozzi, Demartini, Gandolfi and Tinazzi 24 The relevant factors associated with episodic FMD include hyperarousal, anxiety, and a history of trauma and abuse. Hyperarousal is challenging to define in neurological terms but is readily identified in psychiatric illness on the mental status exam (eg, PTSD and anxiety), and we argue is also relevant to the neurological examination, and neuropsychiatric disease in general.Reference Keynejad, Frodl, Kanaan, Pariante, Reuber and Nicholson 13 , Reference Murphy, Nasa and Cullinane 25 , Reference McTeague and Lang 26 Little is understood about how elevated sympathetic tone or a dysregulated stress response modulates the neurological exam, for example, in producing paratonia, altering reflexes, or gating of afferent sensory information during dissociative states. Our results indicate that hyperarousal was a reliable clinical signal strongly associated with trauma and adverse life events, anxiety, and hyperkinetic movements. Multiple studies implicate dysregulated arousal in the pathophysiology of FND.Reference Paredes-Echeverri, Maggio, Bègue, Pick, Nicholson and Perez 12 , Reference Keynejad, Frodl, Kanaan, Pariante, Reuber and Nicholson 13 , Reference Murphy, Nasa and Cullinane 25 Our results suggest that trauma and abuse history appear to be more relevant to the episodic group, although do not predict the development of a specific motor phenotype. Instead, we hypothesize that these experiences become embedded within the developing nervous system and contribute to a dysregulated arousal response, which confers an increased risk for the development and maintenance of some forms of FMD; however, further research in this area is certainly required.Reference Ludwig, Pasman and Nicholson 17 , Reference Lidstone, Nassif, Juncos, Factor and Lang 27 -Reference Apazoglou, Mazzola, Wegrzyk, Polara and Aybek 29
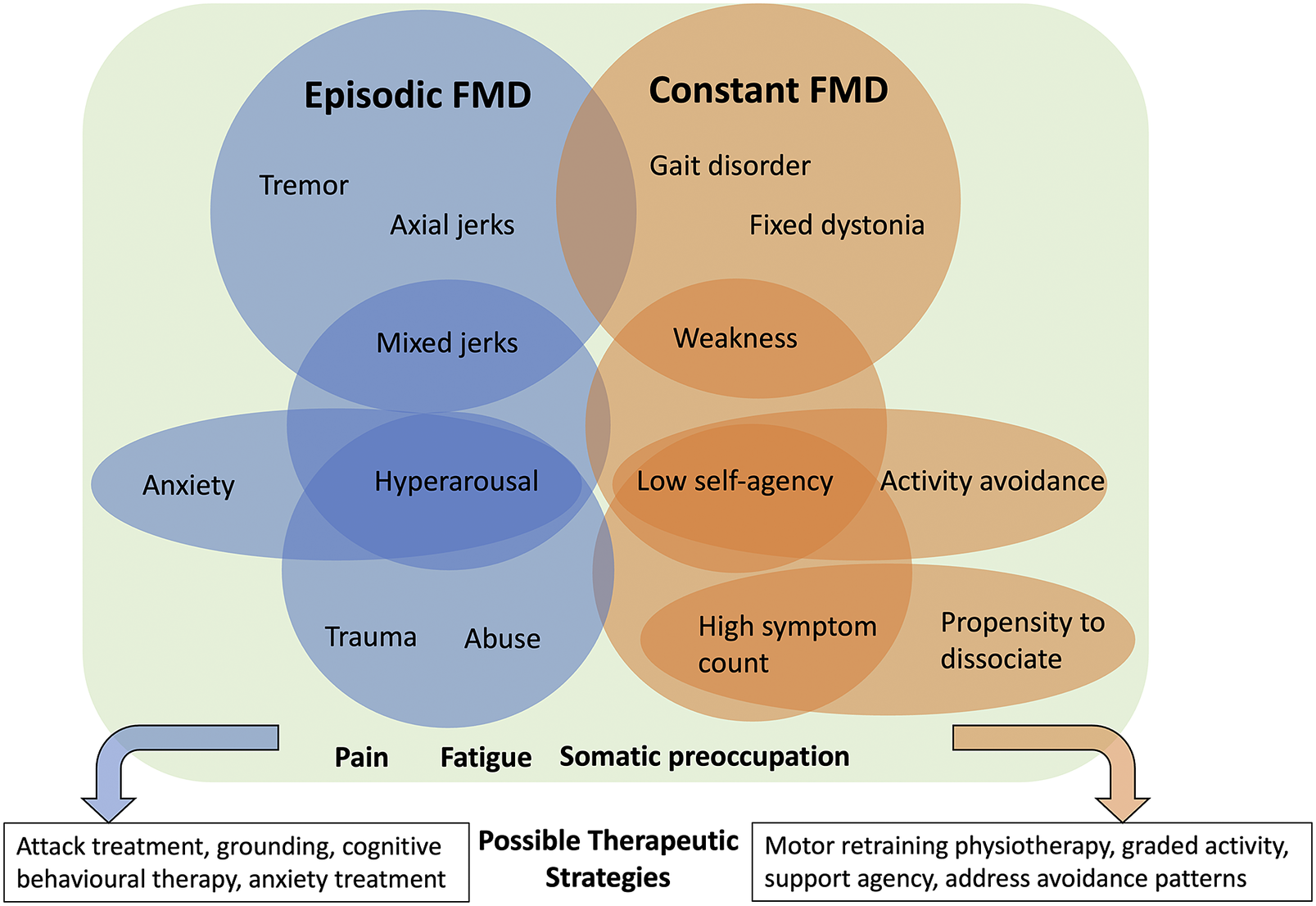
Figure 1. Conceptual figure illustrating new observations and patterns spanning the neurological-psychiatric interface. Episodic and constant FMD are associated with different movement disorder phenotypes and associated psychological characteristics that can inform treatment planning. Pain, fatigue and somatic preoccupation are common to both. Importantly, these patterns may overlap, and not any one factor can be considered etiological. Rather, if present, such factors can be considered potentially relevant as part of a broader FMD syndrome, and targetable with treatment.
The pattern of deficit in constant FMD appears fundamentally different from discrete attacks of hyperkinetic symptoms, with different associated non-motor features. Constant FMD is associated with gait disorders, fixed dystonia, and weakness, as well as low self-agency and activity avoidance, and higher total symptom counts. Clinically, these patients often require gait aids and have prominent fatigue and or pain, and disability related to the constant nature of their symptoms. Motor agency—the ability for one to take ownership of one’s own movement—is a key mechanistic element in FMD in general and localizes to the right temporoparietal junction in multiple studies.Reference Hallett, Aybek, Dworetzky, McWhirter, Staab and Stone 23 In day-to-day life, reduced agency is expanded into a behavior pattern where a lack of control over movement extends to the self or environment, producing a tendency to allow others to provide care needs. Activity avoidance manifests as patterns of reduced participation in activities of daily living and fear of symptom exacerbation, which may be reinforced by prior experience, and lead to kinesiophobia and deconditioning.Reference Maggio, Kyle, Stephen and Perez 11
Identifying neuropsychiatric FMD phenotypes has important treatment implications. In our experience, differentiating episodic and constant FMD is an important early step for treatment planning since they benefit from different approaches to therapy. Although this research is in the early days, episodic FMD symptoms can be successfully treated using strategies focusing on attack treatment and prevention. In contrast, constant FMD symptoms seem more amenable to motor retraining physiotherapy and improving activity tolerance.Reference Nielsen, Stone and Matthews 30 Our results suggest that tailoring treatment plans further to include associated neuropsychiatric factors might enhance recovery potential, given that many of the FMD-relevant factors identified here are also considered perpetuating factors in a biopsychosocial model. For example, in patients with constant FMD, explicitly supporting a sense of self-agency, or identifying and targeting activity avoidance patterns could enhance an individualized physical rehabilitation program. In contrast, patients with episodic FMD could also benefit from the incorporation of anxiety reduction strategies, emotional regulation/dialectic behavioral therapy principles, or boundary setting in parallel. Regardless of phenotype, we would emphasize the critical role of psychoeducation to help patients adopt a non-dualistic view of their symptoms, recognize the role of stress on the nervous system, and the importance of self-management in FND recovery. Treatment specificity and response in FMD requires further research as it is well recognized that one size does not fit all and about 30% of patients remain refractory to treatment.Reference Nielsen, Buszewicz and Stevenson 31 This initial analysis might serve as a first step in identifying additional treatment targets that if present, could result in more tailored—and hopefully effective—therapeutic plans, and further confirm the presence of clinically meaningful neuropsychiatric phenotypes in FMD.
Our sub-specialist database enabled further probing of previously reported clinical FMD features. First, assessment at 2 different time points indicated instability in the motor presentation, with 42% of patients having a change in movement symptoms, irrespective of the duration of symptoms or time between appointments.Reference Tomić, Ječmenica Lukić and Petrović 6 , Reference Ercoli, Tinazzi and Geroin 7 The distinction between episodic and constant symptoms remained somewhat more fixed, reinforcing a possible persistent difference between these groups. Our results also indicate a prominent non-motor syndrome in FMD, that importantly, often precedes the onset of motor symptoms, as is the case in many other movement disorders including Parkinson’s disease.Reference Seppi, Ray Chaudhuri and Coelho 32 , Reference Bhatia and Stamelou 33 Pain, fatigue, and health anxiety were common in all phenotypic clusters, in agreement with previous publications, and persisted in those whose motor symptoms remitted.Reference Gelauff, Rosmalen, Gardien, Stone and Tijssen 4 , Reference Forejtová, Serranová and Sieger 5 Taken together, these findings reinforce the concept of an FMD syndrome that is not sufficiently defined based on motor phenotype alone.Reference Lidstone, Costa-Parke, Robinson, Ercoli and Stone 2 , Reference Gelauff, Rosmalen, Gardien, Stone and Tijssen 4 , Reference Gilmour and Lidstone 34
Our results confirm previous reports of a mean diagnostic delay of 6 years, and invalidating healthcare experiences experienced by patients.Reference Lidstone, MacGillivray and Lang 21 , Reference Aybek, Lidstone and Nielsen 35 We also identified a precipitating trigger for motor symptom onset in 83% of individuals, which is higher than other reports (48%-80%).Reference Perez, Aybek and Popkirov 15 We attribute this to the substantial time spent in the assessment dedicated to understanding the symptom narrative, and considering a broad range of possible triggers. Most patients had greater than one movement disorder (52%), also higher than previously reported (23%).Reference Lidstone, Costa-Parke, Robinson, Ercoli and Stone 2 This may be accounted for by the methods used for this study (ie, combining clinical records and videos to note all symptoms present rather than focusing solely on dominant symptoms). Careful evaluation of patients with appendicular jerks/myoclonus indicated that almost all of them had complex movements that combined jerks with dystonic posturing, tremor, gait disorders, and facial movements, suggesting that “mixed jerks” or “mixed hyperkinetic” may be more appropriate terms. This difficulty in adapting nomenclature used in other movement disorders to FMD has been acknowledged, owing to the fact that abnormal functional movements, by definition, are clinically incongruent with recognized neurological disorders.Reference Gelauff, Rosmalen, Gardien, Stone and Tijssen 4 Rather than trying to fit a “square peg in a round hole,” we advocate instead to recognize FMD phenotypes as consisting of their own unique, positive phenomena.
This study has a number of limitations. The main purpose of this work was to explore patterns in neuropsychiatric symptom expression from a more holistic starting point, without assuming dominance of motor symptoms in the spectrum of FMD presentations. This requires to some extent to approach the problem from a non-siloed viewpoint, which is heavily subjective and dependent on the observer. All patients were assessed by the same clinicians who share a similar perspective on FMD, which may have introduced bias, particularly in terms of how assessments are conducted with attention to FMD-related factors. The list of FMD-related factors is by no means complete, and several others could have been included which are harder to measure and capture (eg, implicit needs being met through illness). Crucially, we do not propose that any of these factors are etiological for FMD. FND is complex and heterogeneous in its development and maintenance, with multiple interrelated aspects of neural function implicated at each stage that are incompletely captured in a biopsychosocial model or formulation. Nor can we say that simply by being associated, these factors are part of the pathophysiology of the disorder. However, the presence of any associations at all supports that observable patterns do exist linking movement and psychological factors that warrant prospective study.
This is a retrospective chart review with a modest sample size, which introduces issues in the data relating to sparse cell counts and numerous relevant symptoms and comorbid medical conditions, and will require larger multi-center prospective research to account for the initial sparse data. Due to limitations inherent to retrospective data, intermittent and paroxysmal symptoms were grouped together and defined as episodic, without differentiating paroxysms/attacks of symptoms vs. symptoms that “come and go.” Now that this initial observation has been made, further refining of episodic vs. constant FMD can occur prospectively. Similarly, a detailed evaluation of the nature of non-motor symptoms including pain and cognitive fog was not done, but should be included in future research. Data were extracted from a subspecialty clinic, and therefore include more complex and persistent FMD cases, underestimating mild and remitted cases. The focus on rehabilitation in the referral pool may have led to the overrepresentation of tremor, jerks, and gait disorders, whereas other movement phenotypes such as fixed dystonia may have been underrepresented. Validated scales were not used to assess functional disability, psychiatric features, trauma history, or FMD-related factors, and require further exploration in prospective studies. Finally, a portion of the data was patient-reported (although also informed by clinical interviews) and liable to recall bias. A validated scale for trauma or adverse life events was not used in this study. In all cases, clinicians did not feel that abnormal functional movement was impacted by medication exposures, but nevertheless medication history, as well as details of comorbid neurological conditions should be included in future studies. Despite these limitations, this study is the first to combine functional movement presentations with psychological and psychiatric factors in a more holistic way, the results of which provide a number of directions for future integrated research.
Conclusion
This hypothesis-generating study provides deep phenotyping of patients across multiple domains of brain function and illness behavior, allowing for the unique exploration of disease expression in FMD. We found significant patterns that suggest that looking beyond the motor phenotype in FMD can reveal associated factors that may be part of recognizable neuropsychiatric phenotypes. Adopting a transdisciplinary view reveals readily identifiable clinical factors as relevant perpetuators of illness, and importantly, potential therapeutic targets. We propose that individual motor presentations of FMD can therefore be “lumped” together, and “split” along more holistic neuropsychiatric lines, which may offer more fruitful avenues for understanding individual mechanisms and treatment. Future interdisciplinary research should aim to further characterize and confirm these episodic and constant phenotypes expanded to include objective and quantitative biomarkers, continue to identify, define, and measure FMD-related factors, and determine if and how such phenotypes inform treatment specificity and success.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S1092852923002353.
Author contribution
Conceptualization: G.S.G., S.C.L.; data curation: G.S.G.; formal analysis: G.S.G., L.K.L., S.C.L.; methodology: G.S.G., L.K.L., S.C.L.; Supervision: S.C.L.; writing—original draft: G.S.G.; writing—reviewing and editing: G.S.G., L.K.L., A.E.L., L.M., S.C.L.
Financial support
This work was supported by an anonymous donation to the Toronto Western Hospital Movement Disorders Clinic.
Disclosure
G.S.G has no disclosures. L.K.L. has no disclosures. A.E.L. has served as an advisor for AbbVie, AFFiRis, BioAdvance, Biogen, BlueRock, BMS, Denali, Janssen, Jazz, Lilly, Paladin, Retrophin, Roche, Sun Pharma, and UCB; received honoraria from AbbVie, Sun Pharma, and Sunovion; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J. Safra Philanthropic Foundation, Michael J. Fox Foundation, Ontario Brain Institute, Parkinson Foundation, Parkinson Canada, and W. Garfield Weston Foundation; received publishing royalties from Cambridge University Press, Elsevier, Johns Hopkins Press, Saunders, and Wiley-Blackwell. L.M. has no disclosures. S.C.L. receives royalties from UpToDate.
Ethical standard
The article received approval from the University Health Network Research Ethics Board (REB 21-6172, approved February 11, 2022).










