Introduction
Unipolar disorder (UD) and bipolar disorder (BD) are among the leading causes of disability worldwide.Reference Whiteford, Degenhardt and Rehm 1 Common features of these mood disorders are persistent cognitive impairments across attention, memory, and executive functionReference Bora, Harrison, Yucel and Pantelis 2 , Reference Bourne, Aydemir and Balanza-Martinez 3 and profound socio-occupational disability.Reference Bonnin, Martinez-Aran and Torrent 4 – Reference Tse, Chan, Ng and Yatham 6 In particular, cognitive impairments directly contribute to patients’ functional disability and high unemployment rates,Reference Bonnin, Martinez-Aran and Torrent 4 – Reference Tse, Chan, Ng and Yatham 6 which constitute the largest socio-economic costs of mood disorders.Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson 7 , Reference Wyatt and Henter 8
Notwithstanding the clear need for treatment to target patients’ cognitive impairments, there are no clinically available treatments with direct pro-cognitive efficacy in mood disorders.Reference Miskowiak, Carvalho, Vieta and Kessing 9 , Reference Miskowiak, Ott, Petersen and Kessing 10 Two recent systematic reviews of cognition trials revealed only preliminary evidence for potential efficacy of candidate treatments in UD and BD, respectively.Reference Miskowiak, Carvalho, Vieta and Kessing 9 , Reference Miskowiak, Ott, Petersen and Kessing 10 The disappointing findings are likely to result from common methodological challenges across cognition trials in mood disorders.Reference Miskowiak, Burdick and Martinez-Aran 11 The International Society for Bipolar Disorder (ISBD) therefore convened an international task force to develop consensus-based recommendations for the design and methodology of cognition trials.Reference Miskowiak, Burdick and Martinez-Aran 11 One of the important task force recommendations was to include neuroimaging assessments in future cognition trials to explore treatment-related target engagement in the neurocircuitries underlying patients’ cognitive impairments. Insights from such assessments may provide a platform for identification of a sensitive neurocircuitry-based biomarker model that can predict treatment efficacy on cognition and thus serve as a surrogate endpoint in treatment development programs.
Converging evidence from preclinical studies and neuroimaging studies in mood disorders suggest that cognitive impairments arise from disruption of neuroplasticity mechanisms and associated functional and structural changes in cognition-relevant neurocircuitries.Reference Carlson, Singh, Jr, Drevets and Manji 12 Specifically, functional magnetic resonance imaging (fMRI) studies have documented aberrant encoding and working memory–related activity in the medial and dorsal prefrontal cortex (PFC), and temporal and parietal regions during acute mood episodes and remission.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 – Reference Townsend, Bookheimer, Foland-Ross, Sugar and Altshuler 20 Patients’ cognitive impairments may also be exacerbated by a failure to suppress neural activity in the default mode network (DMN), a network of medial brain regions implicated in self-referential thoughts and thought wandering.Reference Fernandez-Corcuera, Salvador and Monte 14 , Reference Sheline, Barch and Price 21 In keeping with these findings, emerging evidence from a few recent intervention trials in mood disorders indicates that cognitive improvements are accompanied by neural activity changes in similar fronto-parietal, temporal, and DMN networks.Reference Miskowiak, Macoveanu and Vinberg 22 – Reference Smith, Browning and Conen 25 However, the precise location(s) and direction of the neural activity changes underlying cognitive impairments and cognitive improvements are unclear. The aims of the present systematic review were therefore to delineate (a) the most reliable neural underpinnings of cognitive impairments in mood disorders and (b) the emerging neural basis for direct or indirect cognitive improvements in response to candidate cognition treatments or reduction in mood symptoms, respectively.
Methods
Search strategy
The present systematic review followed the procedures of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.Reference Moher, Liberati, Tetzlaff and Altman 26 Systematic computerized searches were performed in the databases PubMed and PsycInfo from inception up until October 31, 2017 (see detailed search strategy in the Supplementary Material, available online). The title/abstract screening and subsequent full-text screening were performed by the authors. Disagreements were discussed and consensus reached in all cases.
Selection criteria
We included original research articles that examined the neural basis of cognitive impairment and/or improvement as measured with fMRI blood-oxygen-level-dependent (BOLD) techniques in adults with UD or BD irrespective of mood state. We excluded articles that (a) were not original (ie, meta-analyses and reviews), (b) were preclinical, (c) did not utilize the fMRI BOLD signal, (d) did not verify a diagnosis of UD or BD with either the Diagnostic and Statistical Manual of Mental Disorders (DSM) 27 or the International Classification of Diseases (ICD), 28 and (e) examined pediatric/adolescent or geriatric populations. Articles were also excluded if (f) organic disease was present (including neurodegenerative diseases, brain tumors, head trauma, and brain surgery); (g) neuroimaging was not related to performance on an objective neuropsychological test; (h) they involved single-case reports; (i) they only relied on resting state fMRI; or (j) they examined the influence of genetic polymorphisms.
With regard to articles investigating cognitive impairment, we included studies with direct comparisons between UD or BD and healthy control (HC) groups, respectively. Regarding articles examining the neural underpinnings of cognitive improvement, we included trials assessing potential “direct” pro-cognitive effects on an intervention and those in which nonspecific cognitive improvement following symptom reduction was observed. In contrast, we excluded articles that (k) assessed cognitive side-effects of treatments. It was also an inclusion criterion for these trials that fMRI had been conducted both before and after treatment or, in the case of randomized controlled trials, at least after treatment completion.
Results
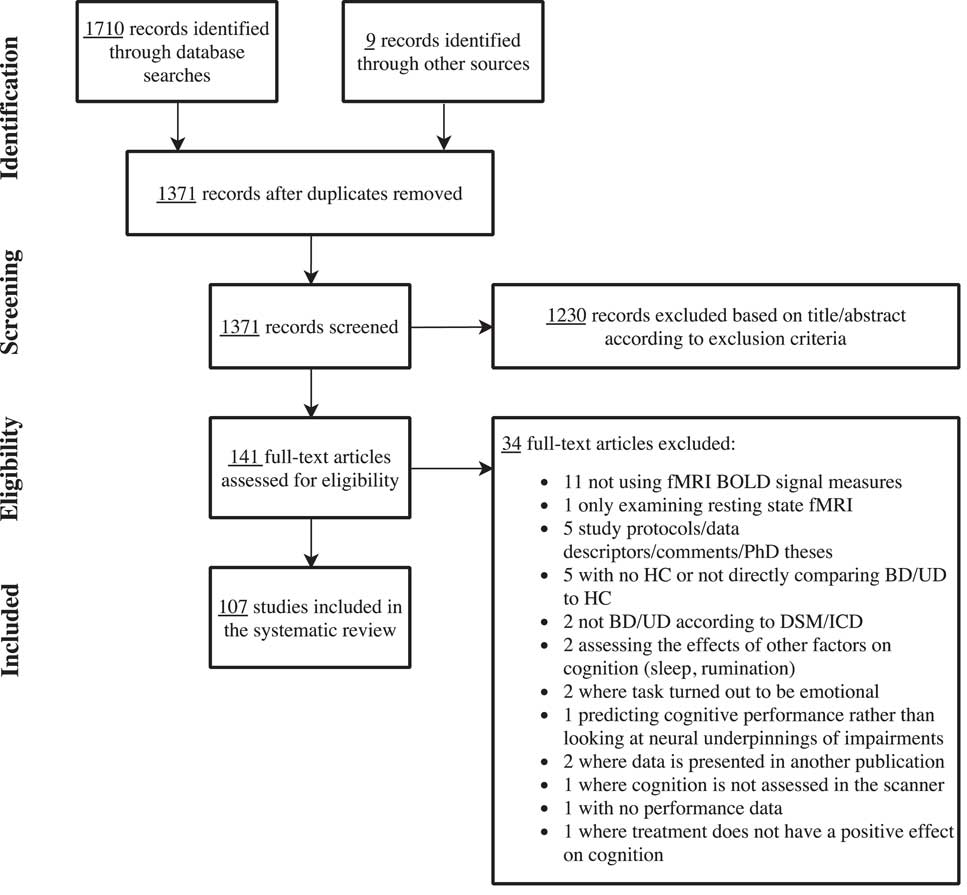
The systematic searches identified a total of 1362 unique articles after deletion of duplicates. An additional 9 references were identified from the reference lists of relevant reviews and meta-analyses. Based on the title/abstract screening, 141 articles were included in the full-text screening. Of these, 107 articles met the specified inclusion criteria and were included in the review. Figure 1 depicts the PRISMA flowchart of the screening procedure. The vast majority of studies (k=100) examined the neural correlates of cognitive impairments, while only 9 studies investigated the neural basis of treatment-related cognitive improvement. Studies of the neural underpinnings of cognitive impairments were grouped into the following cognitive domains based on the employed fMRI paradigms: “working memory,” “executive skills,” “learning and memory,” “attention,” and “implicit learning.” When the neural correlates of more than one relevant fMRI paradigm was reported in the same article, the results for each paradigm was presented under their respective cognitive domain. Similarly, articles investigating the neural correlates of both cognitive impairment and cognitive improvement appear twice in the respective tables.
Cognitive impairment
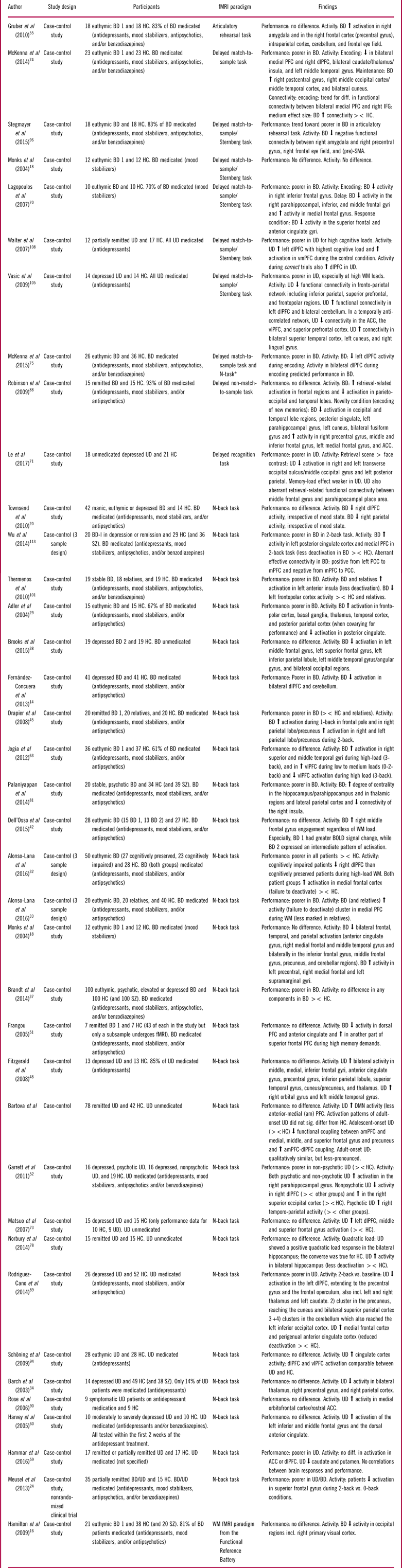
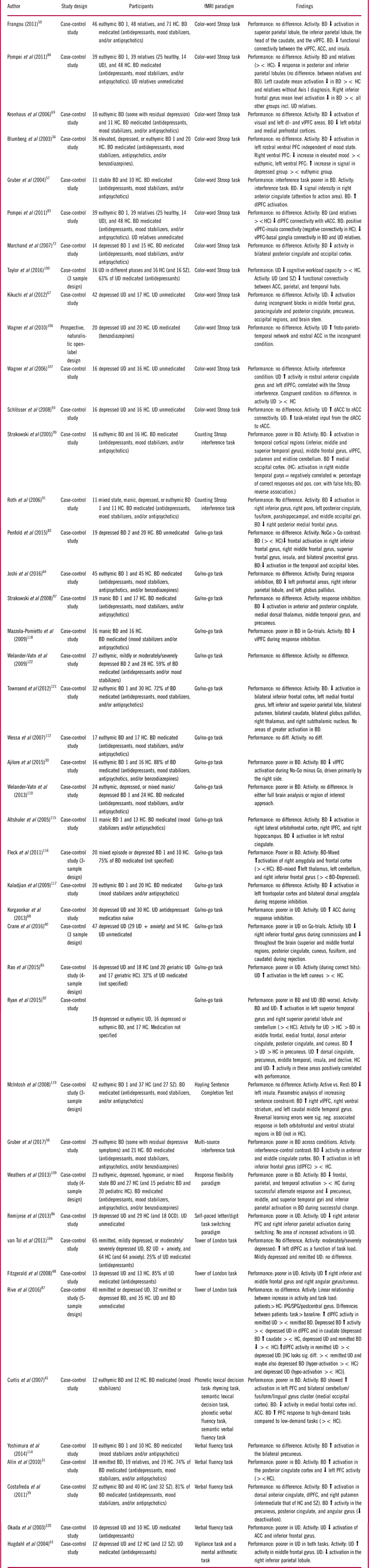
The design and findings of the 100 fMRI studies of cognitive impairments can be seen in Tables 1–3.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Fernandez-Corcuera, Salvador and Monte 14 , Reference Hamilton, Altshuler and Townsend 16 , Reference Monks, Thompson and Bullmore 18 , Reference Townsend, Bookheimer, Foland-Ross, Sugar and Altshuler 20 , Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 , Reference Adler, Holland, Schmithorst, Tuchfarber and Strakowski 29 – Reference Welander-Vatn, Jensen and Lycke 122 The studies were distributed across the domains “working memory” (k=37; Table 1), “executive skills” (k=43; Table 2), “learning and memory” (k=15; Table 3), “attention” (k=6; Table 3), and “implicit learning” (k=2; Table 3).
Table 1 Summary of included studies for the cognitive domain: working memory
Abbreviations: HC: Healthy control, BD: Bipolar disorder, UD: Unipolar disorder, SZ: Schizophrenia, PFC: prefrontal cortex, mPFC: medical PFC, lPFC: lateral PFC, dlPFC: dorsolateral PFC, vmPFC: Ventromedial PFC, amPFC: Anterior medial PFC, SMA: Supplementary motor area, PCC: posterior cingulate cortex, STG: Superior temporal gyrus, WM: Working memory, ><: Compared to.
* The authors combined the results from the two fMRI tasks for an overall working memory score.
Table 2 Summary of included studies for the cognitive domain: executive skills
Abbreviations: HC: Healthy control, BD: Bipolar disorder, UD: Unipolar disorder, SZ: Schizophrenia, OCD: Obsessive compulsive disorder, PFC: prefrontal cortex, dlPFC: dorsolateral PFC, vlPFC: ventrolateral PFC, ACC: Anterior cingulate cortex, vACC: Ventral ACC, rACC: rostral ACC, Anterior cingulate gyrus, IPG: inferior parietal gyrus, SPG: superior parietal gyrus, sig. diff: Significant difference, ><: Compared to.
Table 3 Summary of included studies for the cognitive domains: learning and memory, attention, and implicit learning
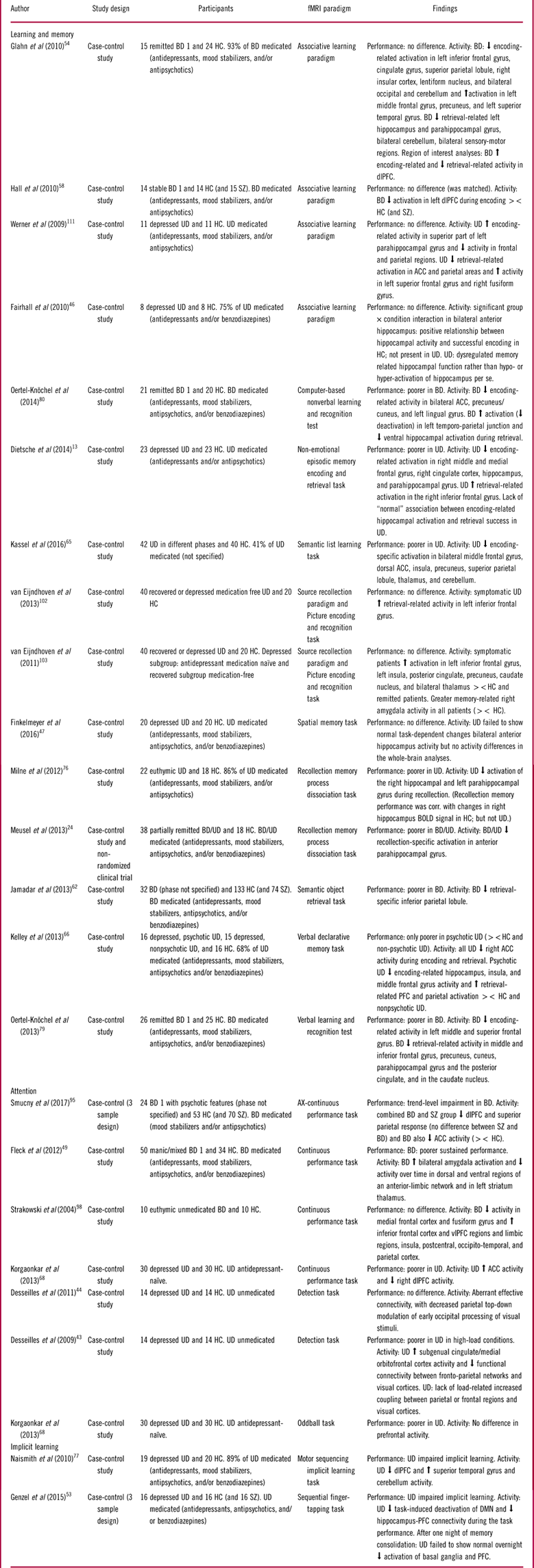
Abbreviations: HC: Healthy control, BD: Bipolar disorder, UD: Unipolar disorder, SZ: Schizophrenia, PFC: prefrontal cortex, dlPFC: dorsolateral PFC, vlPFC: ventrolateral PFC, ACC: Anterior cingulate cortex, sig. diff: Significant difference, ><: Compared to.
Working memory
Neural responses during working memory were assessed in 37 studies using a variety of fMRI paradigms, of which the most common ones were the n-back and delayed match-to-sample/Sternberg tasks. Twenty-two studies involved BD, 14 studies involved UD, and 1 study examined both groups (see Table 1).
Working memory in bipolar disorder
Thirteen (59%) studies reported impaired working memory performance in symptomatic and remitted BD patients (ie, poorer accuracy and/or slowed response times), whereas 9 (41%) studies found no performance impairment. The most robust findings were (i) hypo-activation in prefrontal cognitive control areas including the dorsolateral PFC (dlPFC)Reference Fernandez-Corcuera, Salvador and Monte 14 , Reference Hamilton, Altshuler and Townsend 16 , Reference Townsend, Bookheimer, Foland-Ross, Sugar and Altshuler 20 , Reference Alonso-Lana, Goikolea and Bonnin 32 , Reference Frangou 51 , Reference Lagopoulos, Ivanovski and Malhi 70 , Reference McKenna, Sutherland, Legenkaya and Eyler 74 , Reference McKenna, Theilmann, Sutherland and Eyler 75 and (ii) failure to deactivate regions within the DMN, most consistently the medial PFCReference Alonso-Lana, Goikolea and Bonnin 32 , Reference Alonso-Lana, Valenti and Romaguera 33 , Reference Gruber, Tost and Henseler 55 , Reference Wu, Wang and Mwansisya 113 in BD relative to HC. These activity differences in cognitive control and DMN regions were commonly accompanied by impaired working memory performance.Reference Fernandez-Corcuera, Salvador and Monte 14 , Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 , Reference Alonso-Lana, Goikolea and Bonnin 32 , Reference Alonso-Lana, Valenti and Romaguera 33 , Reference Lagopoulos, Ivanovski and Malhi 70 , Reference McKenna, Sutherland, Legenkaya and Eyler 74 , Reference McKenna, Theilmann, Sutherland and Eyler 75 , Reference Thermenos, Goldstein and Milanovic 101 , Reference Wu, Wang and Mwansisya 113 In keeping with this, a study specifically comparing cognitively impaired with cognitively intact BD patients revealed lower dlPFC activation in the impaired group.Reference Alonso-Lana, Goikolea and Bonnin 32 Another consistent finding was altered fronto-polar cortex activation in remitted BD, with studies indicating increased activation at low task loads and decreased activation at high task load.Reference Adler, Holland, Schmithorst, Tuchfarber and Strakowski 29 , Reference Drapier, Surguladze and Marshall 45 , Reference Jogia, Dima, Kumari and Frangou 63 , Reference Thermenos, Goldstein and Milanovic 101 Dorsolateral PFC hypo-activity thus seems to be linked to lower cognitive capacity (ie, impaired performance), while dlPFC hyper-activity may reflect lower cortical efficiency (ie, having to recruit more neural resources to maintain normal performance). Indeed, Adler et al Reference Adler, Holland, Schmithorst, Tuchfarber and Strakowski 29 found that fMRI analyses co-varied for performance levels revealed task-related hyper-activation of fronto-polar cortex in remitted patients.
Finally, working memory performance in BD was also commonly associated with aberrant functional connectivity (FC) within subcortical and PFC structures as well as between subcortical structures and PFC,Reference McKenna, Sutherland, Legenkaya and Eyler 74 , Reference Palaniyappan and Liddle 81 , Reference Stegmayer, Usher and Trost 96 , Reference Wu, Wang and Mwansisya 113 although the findings regarding the direction of these FC changes were heterogeneous.
Working memory in unipolar disorder
Six (43%) studies found working memory impairments in symptomatic and remitted UD,Reference Garrett, Kelly, Gomez, Keller, Schatzberg and Reiss 52 , Reference Hammar, Neto, Clemo, Hjetland, Hugdahl and Elliott 59 , Reference Le, Borghi, Kujawa, Klein and Leung 71 , Reference Rodriguez-Cano, Sarro and Monte 89 , Reference Vasic, Walter, Sambataro and Wolf 105 , Reference Walter, Wolf, Spitzer and Vasic 108 while 8 (57%) showed comparable performance in UD and HC.Reference Barch, Sheline, Csernansky and Snyder 34 , Reference Bartova, Meyer and Diers 35 , Reference Fitzgerald, Srithiran and Benitez 48 , Reference Harvey, Fossati and Pochon 60 , Reference Matsuo, Glahn and Peluso 73 , Reference Norbury, Godlewska and Cowen 78 , Reference Rose, Simonotto and Ebmeier 90 , Reference Schoning, Zwitserlood and Engelien 94 As with BD, the most consistent neural activation differences during working memory were altered response of cognitive control areas, most consistently in the dlPFCReference Fitzgerald, Srithiran and Benitez 48 , Reference Garrett, Kelly, Gomez, Keller, Schatzberg and Reiss 52 , Reference Harvey, Fossati and Pochon 60 , Reference Matsuo, Glahn and Peluso 73 , Reference Rodriguez-Cano, Sarro and Monte 89 , Reference Walter, Wolf, Spitzer and Vasic 108 and impaired deactivation of DMN regions, including the medial PFC.Reference Bartova, Meyer and Diers 35 , Reference Rodriguez-Cano, Sarro and Monte 89 , Reference Rose, Simonotto and Ebmeier 90
Of 6 studies in symptomatic and partially remitted patients, 2 studies found dlPFC hypo-activation, which was associated with impaired performance,Reference Garrett, Kelly, Gomez, Keller, Schatzberg and Reiss 52 , Reference Rodriguez-Cano, Sarro and Monte 89 while another 4 found dlPFC hyper-activity that was accompanied by preserved performance.Reference Fitzgerald, Srithiran and Benitez 48 , Reference Harvey, Fossati and Pochon 60 , Reference Matsuo, Glahn and Peluso 73 , Reference Walter, Wolf, Spitzer and Vasic 108 However, this association between direction of dlPFC activity and performance was not uniform; 3 studies reported no increase in dlPFC in remitted patients with intact working memory performance,Reference Barch, Sheline, Csernansky and Snyder 34 , Reference Norbury, Godlewska and Cowen 78 , Reference Schoning, Zwitserlood and Engelien 94 and 1 study found no dlPFC hypo-activation in cognitively impaired patients.Reference Hammar, Neto, Clemo, Hjetland, Hugdahl and Elliott 59 Notably, 2 of the 3 studies showing intact working memory performance and normal dlPFC activity were conducted in remitted patients, suggesting that cognitive and neural functioning is normalized after remission in some UD patientsReference Barch, Sheline, Csernansky and Snyder 34 , Reference Schoning, Zwitserlood and Engelien 94 . Finally, symptomatic and remitted UD patients were found to display altered FC within PFC regions and between PFC and parietal nodes of the cognitive control network.Reference Bartova, Meyer and Diers 35 , Reference Le, Borghi, Kujawa, Klein and Leung 71 , Reference Vasic, Walter, Sambataro and Wolf 105
Executive skills
Forty-three studies examined executive skills using a variety of different tasks, most commonly the Stroop, Go/No-Go, Tower of London, and Verbal fluency tasks. Twenty-eight studies were conducted in patients with BD and 13 in patients with UD, while 2 studies examined both populations (see Table 2).
Executive skills in bipolar disorder
Seventeen (61%) studies in BD reported no behavioral differences from HC, while 11 (39%) studies demonstrated poorer performance in BD (see Table 2). Of these, most studies that reported no behavioral differences were conducted in remitted patients.
A highly consistent finding across 75% of the studies was hypo-activity within a distributed cognitive control network including vlPFC/inferior frontal gyrus, dlPFC, and inferior and superior parietal areas, which appeared to be largely independent of mood state and performance levels,Reference Ajilore, Vizueta, Walshaw, Zhan, Leow and Altshuler 30 , Reference Allin, Marshall and Schulze 31 , Reference Blumberg, Leung and Skudlarski 36 , Reference Costafreda, Fu and Picchioni 39 , Reference Frangou 50 , Reference Joshi, Vizueta and Foland-Ross 64 , Reference Kronhaus, Lawrence and Williams 69 , Reference Penfold, Vizueta, Townsend, Bookheimer and Altshuler 82 , Reference Pompei, Jogia and Tatarelli 84 , Reference Roth, Koven and Randolph 91 , Reference Ryan, Dawson and Kassel 92 , Reference Strakowski, Adler, Holland, Mills, DelBello and Eliassen 99 , Reference Weathers, Brotman and Deveney 109 , Reference Altshuler, Bookheimer and Townsend 115 , Reference Kaladjian, Jeanningros, Azorin, Nazarian, Roth and Mazzola-Pomietto 117 , Reference Mazzola-Pomietto, Kaladjian, Azorin, Anton and Jeanningros 118 , Reference Townsend, Bookheimer and Foland-Ross 121 with only 25% of studies reporting hyper-activation in these regions.Reference Curtis, Thompson and Seal 41 , Reference Gruber, Dahlgren and Sagar 56 , Reference Gruber, Rogowska and Yurgelun-Todd 57 , Reference Rive, Koeter, Veltman, Schene and Ruhe 87 , Reference Fleck, Kotwal and Eliassen 116 , Reference McIntosh, Whalley and McKirdy 119 Hypo-activation was particularly pronounced in prefrontal, parietal, and striatal regions.Reference Ajilore, Vizueta, Walshaw, Zhan, Leow and Altshuler 30 , Reference Allin, Marshall and Schulze 31 , Reference Blumberg, Leung and Skudlarski 36 , Reference Frangou 50 , Reference Joshi, Vizueta and Foland-Ross 64 , Reference Kronhaus, Lawrence and Williams 69 , Reference Penfold, Vizueta, Townsend, Bookheimer and Altshuler 82 , Reference Pompei, Jogia and Tatarelli 84 , Reference Roth, Koven and Randolph 91 , Reference Strakowski, Adler, Holland, Mills, DelBello and Eliassen 99 , Reference Weathers, Brotman and Deveney 109 , Reference Altshuler, Bookheimer and Townsend 115 , Reference Kaladjian, Jeanningros, Azorin, Nazarian, Roth and Mazzola-Pomietto 117 , Reference Mazzola-Pomietto, Kaladjian, Azorin, Anton and Jeanningros 118 , Reference Townsend, Bookheimer and Foland-Ross 121 The findings indicate that hypo-activation in the cognitive control network in BD can occur even when the task load does not exceed patients’ cognitive capacity.
Abnormal task-related anterior cingulate cortex (ACC) activity, particularly in the dorsal part, was also reported in multiple BD studies across mood states. Again, the majority of these studies reported hypo-activation,Reference Curtis, Thompson and Seal 41 , Reference Gruber, Dahlgren and Sagar 56 , Reference Gruber, Rogowska and Yurgelun-Todd 57 , Reference Ryan, Dawson and Kassel 92 , Reference Strakowski, Adler and Cerullo 97 , Reference Altshuler, Bookheimer and Townsend 115 while 2 reported hyper-activationReference Costafreda, Fu and Picchioni 39 or no differences.Reference Welander-Vatn, Jensen and Otnaess 110 A final replicated finding was decreased task-related ventral ACC-PFC FC in remitted BD.Reference Frangou 50 , Reference Pompei, Dima, Rubia, Kumari and Frangou 83
Executive skills in unipolar disorder
Nine (69%) studies of UD found impaired task performance,Reference Crane, Jenkins and Dion 40 , Reference Fitzgerald, Srithiran and Benitez 48 , Reference Hugdahl, Rund and Lund 61 , Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68 , Reference Rao, Kassel and Weldon 85 , Reference Remijnse, van den Heuvel and Nielen 86 , Reference Taylor, Theberge, Williamson, Densmore and Neufeld 100 , Reference van Tol, van der Wee and Demenescu 104 , Reference Okada, Okamoto, Morinobu, Yamawaki and Yokota 120 particularly in patients with greater depression severity,Reference van Tol, van der Wee and Demenescu 104 while 4 (31%) studiesReference Kikuchi, Miller and Schneck 67 , Reference Schlösser, Wagner, Koch, Dahnke, Reichenbach and Sauer 93 , Reference Wagner, Koch and Schachtzabel 106 , Reference Wagner, Sinsel and Sobanski 107 reported no behavioral differences. These studies were almost all conducted in symptomatic patients. In general, patients exhibited hypo-activation in prefrontal and parietal cognitive control regions when performance was impaired and hyper-activation in this network when performance was preserved,Reference Crane, Jenkins and Dion 40 , Reference Remijnse, van den Heuvel and Nielen 86 , Reference Rive, Koeter, Veltman, Schene and Ruhe 87 , Reference van Tol, van der Wee and Demenescu 104 , Reference Wagner, Koch and Schachtzabel 106 , Reference Wagner, Sinsel and Sobanski 107 , Reference Okada, Okamoto, Morinobu, Yamawaki and Yokota 120 although 2 studies found hyper-activity in these regions in patients with impaired performance.Reference Fitzgerald, Srithiran and Benitez 48 , Reference Ryan, Dawson and Kassel 92 The areas with most consistent activation abnormalities were inferior, middle, and frontal gyrus; anterior PFC; dlPFC; and inferior parietal cortex. In addition, symptomatic patients also displayed hyper-activity in dorsal and rostral ACC activation,Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68 , Reference Ryan, Dawson and Kassel 92 , Reference Wagner, Koch and Schachtzabel 106 , Reference Wagner, Sinsel and Sobanski 107 although 1 study observed ACC hypo-activation.Reference Okada, Okamoto, Morinobu, Yamawaki and Yokota 120 As in BD, UD patients also showed altered FC within the ACC and between ACC and parietal and temporal hubs.Reference Schlösser, Wagner, Koch, Dahnke, Reichenbach and Sauer 93 , Reference Taylor, Theberge, Williamson, Densmore and Neufeld 100 Finally, a study investigating both UD and BD in depressed or remitted states in comparison with HC reported more pronounced executive deficits in BD than in UD, which were coupled with hypo-activity in BD and hyper-activity in UD.Reference Ryan, Dawson and Kassel 92
Learning and memory
Fifteen studies investigated learning and memory: 5 in BD,Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Hall, Whalley and Marwick 58 , Reference Jamadar, O’Neil and Pearlson 62 , Reference Oertel-Knochel, Reinke and Feddern 79 , Reference Oertel-Knochel, Reinke and Feddern 80 9 in UD,Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Fairhall, Sharma, Magnusson and Murphy 46 , Reference Finkelmeyer, Nilsson and He 47 , Reference Kassel, Rao and Walker 65 , Reference Kelley, Garrett and Cohen 66 , Reference Milne, MacQueen and Hall 76 , Reference van Eijndhoven, van Wingen and Fernandez 102 , Reference van Eijndhoven, van Wingen and Fernandez 103 , Reference Werner, Meindl and Materne 111 and 1 in bothReference Meusel, Hall, Fougere, McKinnon and MacQueen 24 (see Table 3).
Learning and memory in bipolar disorder
Three (60%) studies of remitted BD patients found impaired performance across verbal and nonverbal memory tasks,Reference Jamadar, O’Neil and Pearlson 62 , Reference Oertel-Knochel, Reinke and Feddern 79 , Reference Oertel-Knochel, Reinke and Feddern 80 while the remaining 2 (40%) studies of associative learning reported no performance deficits.Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Hall, Whalley and Marwick 58 Bipolar disorder patients generally exhibited encoding-related hypo-activation in a network of inferior and middle frontal gyrus, ACC, dlPFC, superior parietal lobule, and insula regions in 80% of studies, independent of recall performance,Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Hall, Whalley and Marwick 58 , Reference Oertel-Knochel, Reinke and Feddern 79 , Reference Oertel-Knochel, Reinke and Feddern 80 or a combination of hypo-activation within nodes of this fronto-parietal network and dlPFC hyper-activity.Reference Glahn, Robinson and Tordesillas-Gutierrez 54 During memory retrieval, remitted BD patients also showed primarily hypo-activation in the middle and inferior frontal gyrus and dlPFC,Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Oertel-Knochel, Reinke and Feddern 79 as well as of the hippocampus and parahippocampal gyrus.Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 , Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Oertel-Knochel, Reinke and Feddern 79 , Reference Oertel-Knochel, Reinke and Feddern 80 Finally, one study also found less retrieval-related deactivation of DMN regions including the temporo-parietal junction.Reference Oertel-Knochel, Reinke and Feddern 80
Learning and memory in unipolar disorder
In UD, 5 (56%) studies found no differences on memory performance between HC and symptomatic or remitted UD.Reference Fairhall, Sharma, Magnusson and Murphy 46 , Reference Finkelmeyer, Nilsson and He 47 , Reference van Eijndhoven, van Wingen and Fernandez 102 , Reference van Eijndhoven, van Wingen and Fernandez 103 , Reference Werner, Meindl and Materne 111 The last four (44%) studies reported memory impairment in symptomatic (with and without psychotic features) and remitted patients.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Kassel, Rao and Walker 65 , Reference Kelley, Garrett and Cohen 66 , Reference Milne, MacQueen and Hall 76
Encoding-related hippocampal and parahippocampal hypo-activation were observed in some studies of UD patients with recall deficits.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Kassel, Rao and Walker 65 , Reference Kelley, Garrett and Cohen 66 Moreover, studies showing no behavioral differences generally failed to demonstrate any encoding- and retrieval-related hippocampal hypo-activity.Reference Fairhall, Sharma, Magnusson and Murphy 46 , Reference van Eijndhoven, van Wingen and Fernandez 102 , Reference van Eijndhoven, van Wingen and Fernandez 103 , Reference Werner, Meindl and Materne 111 Another consistent finding in UD patients was the absence of a “normal” association between greater hippocampal and parahippocampal activity during encoding and more subsequent retrieval success.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Fairhall, Sharma, Magnusson and Murphy 46 Another common finding was that patients with poorer memory performance showed encoding-related hypo-activation in the middle and medial frontal gyrus and ACC.Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Kassel, Rao and Walker 65 , Reference Kelley, Garrett and Cohen 66 During memory retrieval, patients were found in several studies to hyper-activate prefrontal structures including the inferior frontal gyrusReference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug 13 , Reference Kelley, Garrett and Cohen 66 , Reference van Eijndhoven, van Wingen and Fernandez 102 , Reference van Eijndhoven, van Wingen and Fernandez 103 and hypo-activate the hippocampus and parahippocampal gyrus.Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 , Reference Kassel, Rao and Walker 65 , Reference Milne, MacQueen and Hall 76
Attention
Six studies examined the neural basis of attention: 3 in BDReference Fleck, Eliassen and Durling 49 , Reference Smucny, Lesh, Newton, Niendam, Ragland and Carter 95 , Reference Strakowski, Adler, Holland, Mills and DelBello 98 and 3 in UD.Reference Desseilles, Balteau and Sterpenich 43 , Reference Desseilles, Schwartz and Dang-Vu 44 , Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68
Attention in bipolar disorder
The 3 fMRI studies of manic and remitted BD patients focused on sustained attention, for which performance was impaired in 2 studiesReference Fleck, Eliassen and Durling 49 , Reference Smucny, Lesh, Newton, Niendam, Ragland and Carter 95 and comparable to HC in one study.Reference Strakowski, Adler, Holland, Mills and DelBello 98 Regardless of performance, BD patients exhibited replicated hyper-activation in limbic structures including the amygdala.Reference Fleck, Eliassen and Durling 49 , Reference Strakowski, Adler, Holland, Mills and DelBello 98 Additionally, patients with performance impairments exhibited hypo-activation of cognitive control regions including the dlPFC, vlPFC, and parietal cortex.Reference Fleck, Eliassen and Durling 49 , Reference Smucny, Lesh, Newton, Niendam, Ragland and Carter 95 In contrast, cognitively intact patients exhibited hyper-activation of the inferior frontal cortex, vlPFC, insula, and parietal regions.Reference Strakowski, Adler, Holland, Mills and DelBello 98
Attention in unipolar disorder
The 3 fMRI studies in symptomatic UD reported reduced sustained and selective attention performance, respectively.Reference Desseilles, Balteau and Sterpenich 43 , Reference Desseilles, Schwartz and Dang-Vu 44 , Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68 Two of these studies found that this deficit was accompanied by aberrant FC between fronto-parietal regions and occipital visual areas, resulting in decreased top-down modulation of early occipital processing.Reference Desseilles, Balteau and Sterpenich 43 , Reference Desseilles, Schwartz and Dang-Vu 44 Korgaonkar et al Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68 also observed dlPFC hypo-activity during sustained attention. In contrast, no abnormal dlPFC activation was detected during a selective attention test despite impaired task performance.Reference Korgaonkar, Grieve, Etkin, Koslow and Williams 68 However, the study involved only region of interest (ROI) analysis, focusing on the dlPFC and dmPFC, and potential abnormalities in other cognitive control regions were thus not assessed.
Implicit learning
Two studies of symptomatic UD investigated implicit learning using a motor sequencing implicit learning task and a sequential finger-tapping task, respectively.Reference Genzel, Dresler and Cornu 53 , Reference Naismith, Lagopoulos, Ward, Davey, Little and Hickie 77 Both found impaired implicit learning in patients, which was accompanied by dlPFC hypo-activity and impaired deactivation of the DMN. Patients also exhibited decreased FC between the hippocampus and PFC.Reference Genzel, Dresler and Cornu 53 In addition, one night of memory consolidation did not result in the “normal” reduction in task-related PFC and basal ganglia activation in UD patients. This could indicate disruption of the brain processes underlying implicit memory consolidation and thus less automation of responses in UD.
Interim summary of cognitive impairment studies
Taken together, the most consistent neural activity changes during working memory, executive skills, memory, and attention domains across BD and UD were abnormal activation of dlPFC, frontopolar, and parietal regions coupled with failure to deactivate the DMN. Patients with BD generally exhibited PFC hypo-activation independent of performance levels, while UD patients generally displayed PFC hypo-activation of these structures when performance was impaired and hyper-activity in this region when performance was preserved. Another common marker of learning difficulties in mood disorders was encoding-related hypo-activation of middle frontal gyrus and ACC. A common phenomenon across the cognitive domains and diagnoses was also the abnormal FC within the PFC regions including the ACC and between PFC and subcortical/parietal regions. In contrast, hippocampal and parahippocampal hypo-activity was shown primarily during memory retrieval in BD and was not consistently observed in patients with UD. Instead, UD patients tended to lack the “normal” correlation between hippocampal engagement during encoding and subsequent retrieval success. Taken together, aberrant (hypo- and hyper-) activity (depending on the level of cognitive performance) in fronto-parietal cognitive control regions and failure to deactivate the DMN may thus represent common fMRI biomarkers of cognitive impairments in mood disorders.
Cognitive improvement
Nine fMRI studies investigated the neural correlates of cognitive improvement in mood disorders, of which 5 focused on specific mood-independent cognitive improvement in response to candidate cognition treatments,Reference Miskowiak, Macoveanu and Vinberg 22 – Reference Smith, Browning and Conen 25 , Reference Haldane, Jogia, Cobb, Kozuch, Kumari and Frangou 123 and 4 investigated nonspecific cognitive improvement following symptom reductionReference Wagner, Koch and Schachtzabel 106 , Reference Kaladjian, Jeanningros and Azorin 124 – Reference Sankar, Adams, Costafreda, Marangell and Fu 126 (see Table 4).
Table 4 Studies of the neural underpinnings of cognitive improvement
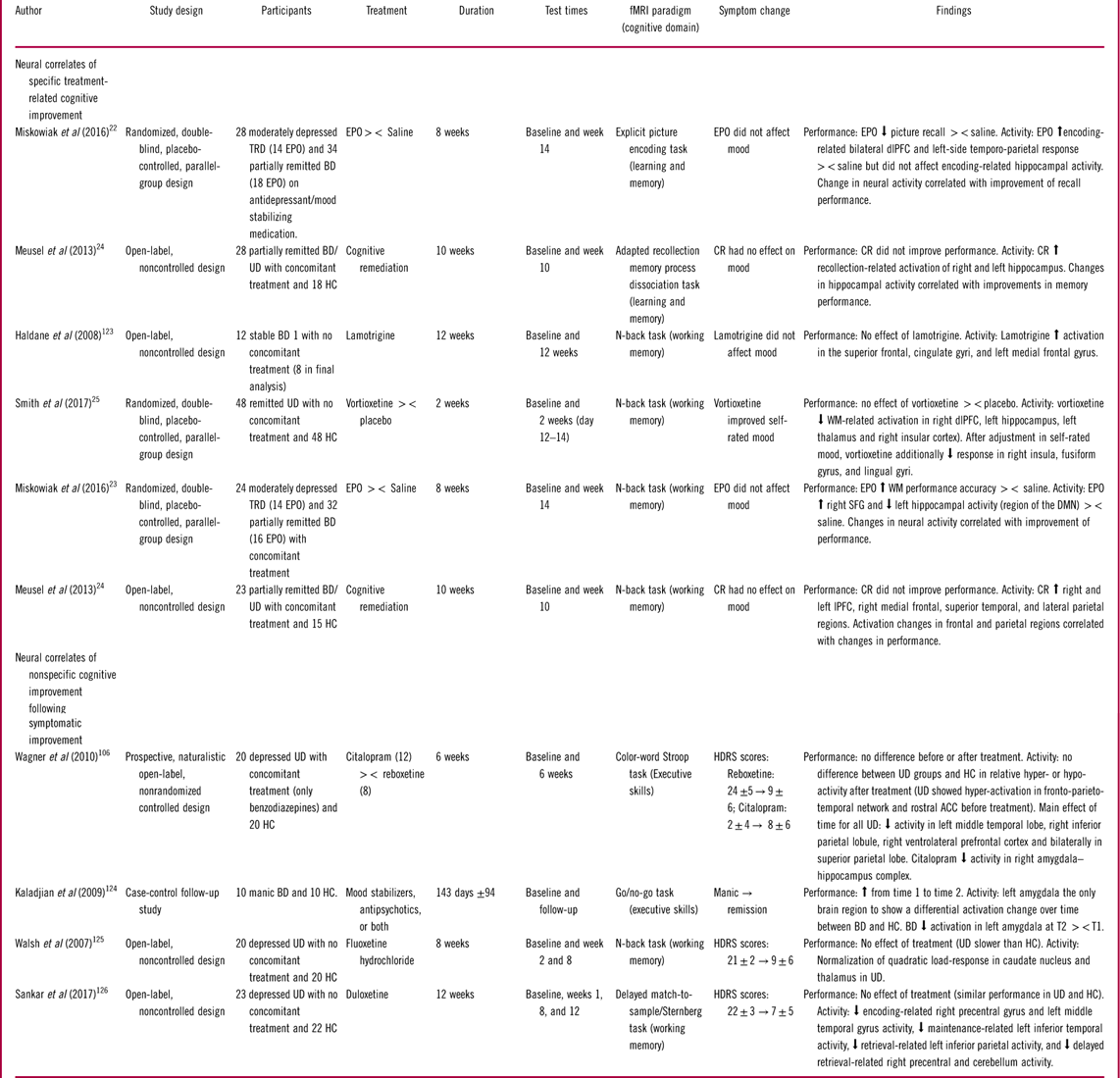
Abbreviations: EPO: Erythropoietin, TRD: Treatment resistant depression, HC: Healthy control, BD: Bipolar disorder, UD: Unipolar disorder, PFC: prefrontal cortex, dlPFC: dorsolateral PFC, lPFC: lateral PFC, SFG: superior frontal gyrus, rACC: Rostral anterior cingulate cortex, WM: Working memory, HDRS: Hamilton depression rating scale, YMRS: Young mania rating scale, BDI: Beck’s depression inventory, SD: Standard deviation, T1: First time point, T2: Second time point, ><: Compared to.
Specific treatment-related cognitive improvement
The 5 studies that investigated specific treatment-related cognitive improvement focused on the effect of 3 different drug treatments [erythropoietin (EPO),Reference Miskowiak, Macoveanu and Vinberg 22 , Reference Miskowiak, Vinberg and Glerup 23 lamotrigine,Reference Haldane, Jogia, Cobb, Kozuch, Kumari and Frangou 123 vortioxetineReference Smith, Browning and Conen 25 ] and one psychological intervention, cognitive remediation (CR) therapy.Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 The treatment-associated changes were investigated on working memory in 4 studies and on learning and memory in 2 studies.
Working memory
Consistent findings regarding the neural correlates of treatment-related improvement of working memory were modulation of task-related activity in the cognitive control regions including the dlPFC and superior frontal gyrusReference Miskowiak, Vinberg and Glerup 23 – Reference Smith, Browning and Conen 25 , Reference Haldane, Jogia, Cobb, Kozuch, Kumari and Frangou 123 and suppression of activity in DMN regions like the hippocampus.Reference Miskowiak, Vinberg and Glerup 23 , Reference Smith, Browning and Conen 25 Specifically, Miskowiak et al Reference Miskowiak, Vinberg and Glerup 23 showed in a randomized placebo-controlled controlled trial (RCT) that 8 weeks of treatment with erythropoietin (EPO) increased working memory capacity in UD and BD, which was accompanied by—and correlated with—enhanced task-related activity in the right superior frontal gyrus and deactivation of the left hippocampus. Similarly, Meusel et al Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 found in an open-label uncontrolled study that CR therapy increased task-related activity in lateral PFC, medial frontal gyrus, superior temporal, and lateral parietal regions. As in the EPO trials, CR-related frontal and parietal activity increase correlated with improved working memory performance. It should be noted, however, that the CR-related working memory improvements did not reach statistical significance, and practice effects could not be excluded given the absence of a control group. The findings regarding the neural correlates of CR should therefore be interpreted with caution.
In contrast to the above-mentioned studies, Smith et al Reference Smith, Browning and Conen 25 found that 2 weeks of treatment with the monoaminergic antidepressant vortioxetine reduced working memory–related dlPFC activity in remitted UD in the absence of changes in working memory performance. Notably, these patients displayed no objective impairment in working memory performance in comparison with HC despite subjective cognitive complaints.Reference Smith, Browning and Conen 25 The reduced dlPFC activity in vortioxetine treated individuals was interpreted as increased cortical efficiency given the absence of overt change in these (cognitively intact) patients’ working memory performance. Indeed, the previously noted distinction between efficiency and capacity is likely to explain the different direction of dlPFC change in response to vortioxetine vs. EPO treatment. Interestingly, vortioxetine also strengthened the deactivation of the hippocampus during working memory performance, similar to the effects of EPO.Reference Miskowiak, Vinberg and Glerup 23
Finally, a small open-label, noncontrolled study of lamotrigine treatment in remitted BD patients revealed increased working memory–related activation over time in bilateral superior frontal and cingulate gyri and left medial frontal gyrus in the absence of changes in performance.Reference Haldane, Jogia, Cobb, Kozuch, Kumari and Frangou 123 However, it is difficult to determine whether this represents a beneficial effect on the neural activity associated with cognitive performance given (i) that the direction of activity change was opposite to the hypothesized (reduced) activity associated with greater efficiency as seen after vortioxetine treatment, (ii) the lack of associated cognitive improvement as seen after EPO treatment, and (iii) the within-group design with no control group, which could not exclude nonspecific effects of repeated testing and learning over time.
Learning and memory
Hippocampus and dlPFC were reported to underlie treatment-related improvements in the 2 studies of learning and memory.Reference Miskowiak, Macoveanu and Vinberg 22 , Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 In the RCT by Miskowiak et al,Reference Miskowiak, Macoveanu and Vinberg 22 EPO treatment increased encoding-related bilateral dlPFC and left-sided temporo-parietal response across BD and UD patients and improved subsequent recall performance. Importantly, the EPO-associated activity increase in dorsal PFC and temporo-parietal regions correlated with improvement of recall performance across the entire cohort, suggesting that this effect was mechanistically important.Reference Miskowiak, Macoveanu and Vinberg 22 In contrast, no treatment-associated change in hippocampal response during memory encoding was observed.Reference Miskowiak, Macoveanu and Vinberg 22 This contrasts with the finding by Meusel et al Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 of CR-related increase in hippocampus during retrieval. However, given the within-group design with no control group in the CR trial, the hippocampal activity increase over time could reflect nonspecific effects of repeated testing rather than specific effects of the intervention.
Cognitive improvement following symptom reduction
Four studies examined improvements in working memory and executive skills following reduction in mood symptoms, of which 3 studies were conducted in depressed UD patientsReference Wagner, Koch and Schachtzabel 106 , Reference Walsh, Williams and Brammer 125 , Reference Sankar, Adams, Costafreda, Marangell and Fu 126 and one in manic BD patients.Reference Kaladjian, Jeanningros and Azorin 124
In general, cognitive improvement following symptom reduction was associated with decreased activation both within cognitive control and DMN regions.Reference Wagner, Koch and Schachtzabel 106 , Reference Kaladjian, Jeanningros and Azorin 124 , Reference Sankar, Adams, Costafreda, Marangell and Fu 126 Indeed, Kaladjian et al Reference Kaladjian, Jeanningros and Azorin 124 found that improved cognitive performance in BD patients after transition from a manic to a remitted state was accompanied by decrease in left amygdala activation during an inhibitory control task. Similarly, Wagner et al Reference Wagner, Koch and Schachtzabel 106 found that reduction in depressive symptoms in UD patients after successful citalopram treatment was accompanied by decreased activity in the amygdala–hippocampus complex during color-word Stroop performance. Successful citalopram and reboxetine treatment of UD patients also attenuated pre-treatment hyper-activation of the fronto-parieto-temporal network and rostral ACC during a cognitive control task.Reference Wagner, Koch and Schachtzabel 106 In contrast, task-related caudate nucleus and thalamus activity increase has also been observed in UD patients after reduction in depressive symptoms.Reference Walsh, Williams and Brammer 125
Interim summary of cognitive improvement studies
Different pharmacological and psychological treatments that directly target cognition seem to specifically modulate dorsal PFC activity—with the direction of this activity change depending on the associated changes in performance levels—and to attenuate DMN hyper-activity. The observed opposite effects of EPO and vortioxetine on working memory-related dlPFC activity may be explained by the associated changes in capacity (ie, performance increase) or efficiency (with no associated behavioral change), respectively. Further, a common neural activity change observed after EPO and CR treatments was increase in task-related dlPFC activity. In contrast, encoding-related hippocampal activity was not modulated by EPO, and it is unclear whether the observed hippocampal activity increase after CR represented a treatment effect or nonspecific changes with repeated testing. Further, indirect cognitive improvement following symptom reduction was consistently accompanied by reduced limbic and DMN activity and reversal of pre-treatment fronto-parietal hyper-activity during task performance. This may represent reduced interference from hyper-active task-negative (limbic and DMN) regions after attenuation of mood symptoms and, consequently, less need for compensatory over-activation in cognition relevant regions.
Discussion
This systematic review examined the most consistent neural correlates for cognitive impairments and cognitive improvement in mood disorders to identify putative neurocircuitry-based targets for novel cognition treatments. We identified a total of 100 studies of the neuronal underpinnings of working memory, executive skills, learning and memory, attention, and implicit learning, respectively, and 9 studies of the neuronal changes associated with cognitive improvements. The most consistent findings regarding neural correlates for cognitive impairments across domains and diagnoses were aberrant (hypo- or hyper-) activity in medial and dorsal PFC cognitive control regions and parietal cortex, with the direction of this aberrant activity depending on cognitive performance levels as well as hyper-activity in the DMN and limbic regions. Candidate treatments that directly targeted cognition seemed to (i) specifically modulate dorsal PFC and temporo-parietal activity, with the direction of the activity change depending on whether it was accompanied by improved cognitive performance, and (ii) attenuate DMN hyper-activity. In contrast, indirect cognitive improvements following symptom reduction were commonly accompanied by attenuation of limbic hyper-reactivity coupled with reversal of pre-treatment fronto-parietal hyper-activity during cognitive performance.
Putative biological targets for pro-cognitive treatments
Remarkably, a few common brain regions were consistently identified as showing abnormal activity in UD and BD across a variety of fMRI paradigms tapping into different cognitive domains. Specifically, fMRI studies of working memory, executive skills, memory encoding, and sustained attention revealed reliable evidence for aberrant (predominantly hypo-) activity in dorsal PFC and fronto-polar regions as well as abnormal FC within the PFC and between the PFC, parietal, and limbic regions. Notably, the dorsal and lateral areas of the PFC are involved in a variety of “top-down” control processes that may be important across several cognitive domains, including active working memory maintenance and manipulation, attention control and -switching, impulse inhibition, and strategic encoding.Reference Ragland, Laird, Ranganath, Blumenfeld, Gonzales and Glahn 19 This may explain the association between aberrant activity in these regions and impaired performance across diverse neurocognitive tests. Another consistent finding across fMRI studies of working memory, executive skills, and attention was reduced deactivation of the DMN and limbic structures during active task performance. This is in line with the hypothesis that cognitive impairments in mood disorders may exacerbated by a failure to suppress task-irrelevant neural activity associated with emotional reactivity, self-focus, and rumination.Reference Fernandez-Corcuera, Salvador and Monte 14 , Reference Sheline, Barch and Price 21 While abnormal hippocampal activity during memory retrieval was a reliable finding in BD patients,Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24 , Reference Glahn, Robinson and Tordesillas-Gutierrez 54 , Reference Oertel-Knochel, Reinke and Feddern 79 , Reference Oertel-Knochel, Reinke and Feddern 80 it was not consistently observed in UD patients.Reference Fairhall, Sharma, Magnusson and Murphy 46 , Reference van Eijndhoven, van Wingen and Fernandez 102 , Reference van Eijndhoven, van Wingen and Fernandez 103 , Reference Werner, Meindl and Materne 111 However, several studies found that UD patients failed to display the “normal” correlation between encoding-related hippocampal activation and subsequent retrieval success. This points to dysregulated encoding-related recruitment of the hippocampus rather than hippocampal hypo- or hyper-activity per se. Taken together, abnormal (predominantly hypo-) activity in dorsal and lateral PFC, aberrant PFC FC, and failure to suppress DMN activity emerged as the most consistent neural correlates of cognitive impairments across UD and BD and therefore represent the most promising biological targets for pro-cognitive interventions.
Efficiency vs. capacity: the importance of performance levels
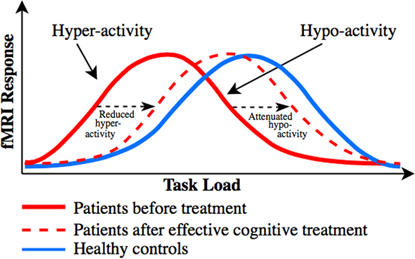
The discrepant findings regarding the direction of abnormal task-related activity in dorsal PFC (particularly in UD) and of the dorsal PFC activity change in response to pro-cognitive interventionsReference Miskowiak, Macoveanu and Vinberg 22 , Reference Miskowiak, Vinberg and Glerup 23 , Reference Smith, Browning and Conen 25 are best explained in relation to patients’ levels of cognitive performance. Specifically, dorsal PFC hyper-activity has been proposed to reflect reduced cortical efficiency, that is, the need for recruitment of more neural resources to maintain normal performance,Reference Callicott, Egan and Mattay 127 whereas dorsal PFC hypo-activity is accompanied by reduced cognitive capacity, that is, performance decline when the task load exceeds individuals’ cognitive capacity.Reference Callicott, Egan and Mattay 127 . Callicott et al Reference Callicott, Egan and Mattay 127 proposed that the dorsal PFC hyper- and hypo-activity in patients can be explained by a leftward shift in the generally observed inverted U-shaped response curve between the cognitive task load and dorsal PFC activity. Specifically, neuropsychiatric patients may reach the peak BOLD response faster (ie, at a lower cognitive load) than HC, after which their dorsal PFC activity and associated performance success go down when the task load exceeds patients’ cognitive capacityReference Callicott, Egan and Mattay 127 (for illustration, see our revised model based on Callicott et al Reference Callicott, Egan and Mattay 127 in Figure 2). Indeed, we observed consistent evidence in this systematic review for dorsal PFC hypo-activity across BD and UD patients who showed impaired cognitive performance in comparison with HC, whereas patients who maintained normal performance levels were commonly characterized by dorsal PFC hyper-activity. Also consistent with this model is the suggestion that frontopolar hypo-activation in BD results from the cognitive load exceeding patients’ capacity to activate this region, which leads to deterioration of their task performance.Reference Jogia, Dima, Kumari and Frangou 63 Further, co-variation for performance levels in another study resulted in frontopolar hyper- (rather than hypo-) activation in BD.Reference Adler, Holland, Schmithorst, Tuchfarber and Strakowski 29 Nevertheless, 2 studies failed to show such an association between hypo-activity and reduced cognitive capacity; instead they found dorsal PFC hyper-activation in patients with executive dysfunction.Reference Fitzgerald, Srithiran and Benitez 48 , Reference Ryan, Dawson and Kassel 92 Given this, task-related PFC hyper-activity may also result from unsuccessful attempts to maintain normal task performance.
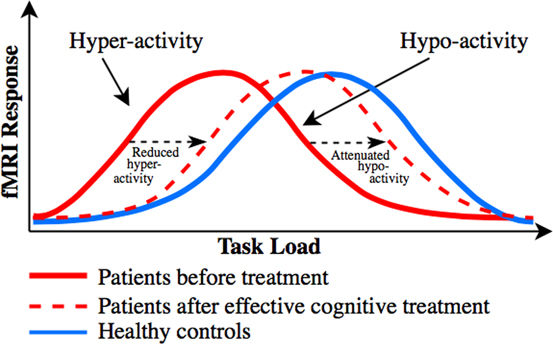
Figure 2 Putative distinct load-response curves, which may unify the discrepant findings regarding dorsal PFC activity change associated with cognitive impairment and cognitive improvement in mood disorders. Model revised from Callicott et al.Reference Callicott, Egan and Mattay 127 Distinct inverted curves for the association between task load (task difficulty) and dorsal PFC activity in patients with neuropsychiatric disorders (red solid curve) and healthy controls (blue solid curve), and distinct changes in task-related dorsal PFC activity in response to treatments targeting cognition. Patients tend to display dorsal PFC hyper-activity in comparison with healthy controls when performance is maintained at medium task loads and dorsal PFC hypo-activity when performance declines at higher loads (where the task demand exceeds patients’ cognitive capacity). We hypothesize that pro-cognitive treatments of patients shift the bell-shaped curve toward the right (ie, toward “normality”), as indicated with the red dotted curve. Depending on the cognitive task load and hence patients’ performance levels, this rightward shift will be reflected by either (A) reduction in pre-treatment dorsal PFC hyper-activity in (cognitively intact) patients who display no treatment-related change in performance (ie, increased cortical efficiency), such as seen after vortioxetine treatment,Reference Smith, Browning and Conen 25 or (B) attenuation of pre-treatment dorsal PFC hypo-activity (ie, enhanced dorsal PFC response) in cognitively impaired patients who display treatment-related cognitive improvement (ie, enhanced cognitive capacity), as seen after erythropoietinReference Miskowiak, Macoveanu and Vinberg 22 , Reference Miskowiak, Vinberg and Glerup 23 and cognitive remediation treatments.Reference Meusel, Hall, Fougere, McKinnon and MacQueen 24
Consistent with the notion that abnormal dorsal PFC and DMN activity may be common biological targets for cognition treatments, the identified cognition trials revealed common treatment-related modulation of activity in these networks. In particular, EPO and CR increased task-related dorsal PFC and parietal activity, which correlated with increased recall performance. Further, modulation of working memory associated dlPFC activity was a common neural correlate of EPO and vortioxetine treatments, although the direction of this change differed between the interventions. We hypothesize that the apparent discrepancy regarding the direction of the dorsal PFC change can be explained by a common treatment-related rightward shift toward “normality” in the putative bell-shaped response curve between task load and dorsal PFC responseReference Callicott, Egan and Mattay 127 (for illustration, see Figure 2). Depending on the cognitive task load and hence patients’ performance levels, this rightward shift will be reflected by either (A) reduction in pre-treatment dorsal PFC hyper-activity in (cognitively intact) patients who display no treatment-related change in performance (ie, increased cortical efficiency), such as seen after vortioxetine treatment,Reference Smith, Browning and Conen 25 or (B) attenuation of pre-treatment dorsal PFC hypo-activity (ie, enhanced dorsal PFC response) in cognitively impaired patients who display treatment-related cognitive improvement (ie, enhanced cognitive capacity), as seen after EPOReference Miskowiak, Macoveanu and Vinberg 22 , Reference Miskowiak, Vinberg and Glerup 23 and CR treatmentsReference Meusel, Hall, Fougere, McKinnon and MacQueen 24 (see Figure 2).
Remarkably, meta-analytical findings point to similar increase in task-related dlPFC and medial PFC activity as the most robust markers of cognitive improvements following CR treatments in schizophrenia.Reference Ramsay and MacDonald 128 In contrast, CR-related activity change in other cognition-relevant regions, such as the hippocampus, was less consistent across schizophrenia trials.Reference Ramsay and MacDonald 128 This is consistent with the lack of reliable treatment effects on encoding-related hippocampal activity in mood disorders. Together, these findings point to modulation of dorsal PFC and the DMN as the most promising surrogate marker of pro-cognitive effects of both pharmacological and cognitive treatments across several neuropsychiatric disorders.
Studies of indirect cognitive improvement following reduction in mood symptoms yielded a somewhat different pattern of neural changes: decrease in hyper-activity in limbic and DMN coupled with reversal of pre-treatment fronto-parietal hyper-activity. Such indirect cognitive improvement could thus be mediated primarily by decreased interference from limbic and DMN hyper-reactivity in parallel with patients’ symptom reduction and consequent “relaxation” of the compensatory hyper-activity in cognitive control regions.
Methodological challenges and opportunities
A greater proportion of studies in UD than in BD patients displayed performance deficits on fMRI paradigms tapping into executive function, which contrasts with evidence for greater severity of cognitive deficits in BD.Reference Gualtieri and Morgan 129 A likely explanation is that fMRI paradigms are generally not optimized for detection of deficits in cognitive performance but for detection of compensatory neural responses associated with intact cognitive performance.Reference Price and Friston 130 Nevertheless, the differential difficulty levels of the employed fMRI paradigms, together with patients’ cognitive heterogeneity, may explain the common fronto-parietal hyper-activity in patients with intact cognitive performance and hypo-activity in those with compromised performance.
Functional MRI can provide a valuable dynamic measure of the treatment effects at a systems level in the brain, which may have better predictive validity than animal models. However, there are some fundamental limitations of the fMRI technique that must be considered in relation to its implementation in treatment development strategies targeting cognition. First, the reproducibility of the BOLD fMRI response is uncertain given inconsistent test–retest reliability across different assessment times in the same individuals.Reference Zandbelt, Gladwin and Raemaekers 131 This limits the statistical power for detection of a treatment effect in fMRI studies with a repeated-measures design. Secondly, the fMRI BOLD response provides only an indirect measure of neural activity. This may be problematic for demonstrating neurocircuitry “target engagement” in response to treatments that influence global cerebral hemodynamic responses.Reference Zandbelt, Gladwin and Raemaekers 131 Indeed, this turned out to be a problem in the EPO studies, since long-term EPO administration upregulates the level of red blood cells. Nevertheless, the problem was tackled by (i) postponing the post-treatment fMRI scan until the red blood cell counts had normalized (and verifying this with blood tests), and (ii) inclusion of a visual stimulation control task with no cognitive demands to examine whether there were any potential global (cognition-unrelated) differences in neural activity between EPO and saline groups.Reference Miskowiak, Macoveanu and Vinberg 22 , Reference Miskowiak, Vinberg and Glerup 23 The golden standard approach would be to apply arterial spin labeling, which is an even more rigorous measure, to quantify and adjust for any potential physiological effects on global hemodynamic responses. Nevertheless, it may be argued that the fMRI BOLD response cannot provide a robust, reliable marker of treatment efficacy because the understanding of its biological basis is incomplete. Indeed, there is a lack of consensus in the field on whether treatment-related increase or decrease in fMRI BOLD is a marker of cognitive improvement. We propose a model that may explain these discrepant findings and become useful for interpretation of neuroimaging findings in future cognition trials (Figure 2). Specifically, the model involves consideration not only of the treatment-related change in dorsal PFC activity but also of the accompanying change in cognitive performance (or lack thereof) for the interpretation of the observed effects. Hence, assessment of treatment-related change in fMRI BOLD signal within key neurocircuitries together with change in cognition is a promising strategy for determining the functional relevance of any neural activity changes.
Conclusion
In conclusion, the most consistent neural underpinnings of cognitive impairments across cognitive domains and diagnoses were aberrant activity in the medial and dorsal PFC cognitive control regions and parietal cortex, with the direction of the aberrant activity depending on patients’ cognitive performance levels. Another common finding was the failure to suppress DMN and limbic activity during cognitive performance. The findings from the cognition trials indicated that the most consistent biological targets for treatments with direct efficacy on cognition are (i) enhancement of activity in dorsal PFC cognitive control regions in patients with impaired cognitive performance (ie, increased capacity) or reduction of neural activity in these regions in patients with intact performance (ie, increased efficiency) (findings that may be reconciled with our proposed model for a rightward shift in the putative bell-shaped curve for the association between BOLD fMRI response and cognitive load; Figure 2), as well as (ii) suppression of activity in the DMN during cognitive performance. In contrast, indirect cognitive improvement following symptom reduction seemed to be mediated by decrease in limbic reactivity coupled with attenuation of fronto-parietal hyper-activity during cognitive performance. This review and integration of the findings in the field provide a first step toward a more unified understanding of the shared neural correlates of cognitive deficits in mood disorders and of treatment-associated cognitive improvements. These insights can provide a platform for studies assessing the predictive validity and reliability of treatment-related modulation of the dorsal PFC and DMN as surrogate markers for pro-cognitive effects. The perspective is the identification of a neurocircuitry biomarker model for pro-cognitive effects that can become a key tool to inform go/no-go decisions before the conduct of large-scale clinical efficacy trials in future treatment development programs.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852918001062