Article contents
Stability of Magadiite Between 20 and 100°C
Published online by Cambridge University Press: 01 January 2024
Abstract
New experimental data with respect to the solubility of natural and synthesized magadiite at elevated temperatures and in alkaline solutions are presented. The results show that the solubility of magadiite increases according to the expression ln(Kmag) = −8146·T(K)−1 − 5.71 from 20 to 100°C ((ΔGR0(Kmag)=19.6±0.3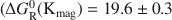 and ΔHR0(Kmag)=16.2±0.4kcalmol−1
and ΔHR0(Kmag)=16.2±0.4kcalmol−1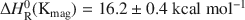 ). The experimental results and the related data from the literature suggest that the formation of magadiite may be favored by a decrease of temperature and pH (pH >9) as well by large amounts of Na+ ions and low ionic strength. These effects are related to the value of ΔHR0(Kmag)
). The experimental results and the related data from the literature suggest that the formation of magadiite may be favored by a decrease of temperature and pH (pH >9) as well by large amounts of Na+ ions and low ionic strength. These effects are related to the value of ΔHR0(Kmag)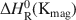 , the distribution of dissolved silica species, the stoichiometry of magadiite, and the occurrence of negatively-charged species, respectively.
, the distribution of dissolved silica species, the stoichiometry of magadiite, and the occurrence of negatively-charged species, respectively.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2002, The Clay Minerals Society
References
- 6
- Cited by




