Article contents
Role of Microbial Fe(III) Reduction and Solution Chemistry in Aggregation and Settling of Suspended Particles in the Mississippi River Delta Plain, Louisiana, USA
Published online by Cambridge University Press: 01 January 2024
Abstract
River-dominated delta areas are primary sites of active biogeochemical cycling, with productivity enhanced by terrestrial inputs of nutrients. Particle aggregation in these areas primarily controls the deposition of suspended particles, yet factors that control particle aggregation and resulting sedimentation in these environments are poorly understood. This study was designed to investigate the role of microbial Fe(III) reduction and solution chemistry in aggregation of suspended particles in the Mississippi Delta. Three representative sites along the salinity gradient were selected and sediments were collected from the sediment-water interface. Based on quantitative mineralogical analyses 88–89 wt.% of all minerals in the sediments are clays, mainly smectite and illite. Consumption of SO42−\$\end{document}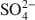 and the formation of H2S and pyrite during microbial Fe(III) reduction of the non-sterile sediments by Shewanella putrefaciens CN32 in artificial pore water (APW) media suggest simultaneous sulfate and Fe(III) reduction activity. The pHPZNPC of the sediments was ⩽3.5 and their zeta potentials at the sediment-water interface pH (6.9–7.3) varied from −35 to −45 mV, suggesting that both edges and faces of clay particles have negative surface charge. Therefore, high concentrations of cations in pore water are expected to be a predominant factor in particle aggregation consistent with the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory. Experiments on aggregation of different types of sediments in the same APW composition revealed that the sediment with low zeta potential had a high rate of aggregation. Similarly, addition of external Fe(II) (i.e. not derived from sediments) was normally found to enhance particle aggregation and deposition in all sediments, probably resulting from a decrease in surface potential of particles due to specific Fe(II) sorption. Scanning and transmission electron microscopy (SEM, TEM) images showed predominant face-to-face clay aggregation in native sediments and composite mixtures of biopolymer, bacteria, and clay minerals in the bioreduced sediments. However, a clear need remains for additional information on the conditions, if any, that favor the development of anoxia in deep- and bottom-water bodies supporting Fe(III) reduction and resulting in particle aggregation and sedimentation.
and the formation of H2S and pyrite during microbial Fe(III) reduction of the non-sterile sediments by Shewanella putrefaciens CN32 in artificial pore water (APW) media suggest simultaneous sulfate and Fe(III) reduction activity. The pHPZNPC of the sediments was ⩽3.5 and their zeta potentials at the sediment-water interface pH (6.9–7.3) varied from −35 to −45 mV, suggesting that both edges and faces of clay particles have negative surface charge. Therefore, high concentrations of cations in pore water are expected to be a predominant factor in particle aggregation consistent with the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory. Experiments on aggregation of different types of sediments in the same APW composition revealed that the sediment with low zeta potential had a high rate of aggregation. Similarly, addition of external Fe(II) (i.e. not derived from sediments) was normally found to enhance particle aggregation and deposition in all sediments, probably resulting from a decrease in surface potential of particles due to specific Fe(II) sorption. Scanning and transmission electron microscopy (SEM, TEM) images showed predominant face-to-face clay aggregation in native sediments and composite mixtures of biopolymer, bacteria, and clay minerals in the bioreduced sediments. However, a clear need remains for additional information on the conditions, if any, that favor the development of anoxia in deep- and bottom-water bodies supporting Fe(III) reduction and resulting in particle aggregation and sedimentation.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © 2008, The Clay Minerals Society
References
- 18
- Cited by




