Article contents
Reactivity of Basal Surfaces, Steps and Edges of Muscovite: An AFM Study
Published online by Cambridge University Press: 28 February 2024
Abstract
The reactivity of basal surfaces, steps and edges of muscovite was studied by imaging surface precipitates of PbCl2 using atomic force microscopy (AFM). We reacted PbCl2 solution with freshly cleaved muscovite surfaces and found that PbCl2 precipitates were formed on the basal surfaces, steps and edges. It was observed that PbCl2 precipitated preferentially along the steps compared to the basal surfaces and that PbCl2 precipitates at multiple-layer edges were needle-shaped and oriented in different directions. One of the muscovite samples we cleaved had muscovite fragments sitting on the freshly cleaved surfaces. These fragments resulted from previously formed cracks. Thus, we were able to compare the reactivity of the weathered surfaces with that of freshly cleaved surfaces. It was found that PbCl2 was not precipitated along the edges of previously cracked muscovite fragments. These results clearly demonstrated that the edges of freshly cleaved muscovite are the most reactive surface sites, whereas the edges of weathered muscovite are not as reactive. We believe that the surface reactivity of the edges of freshly cleaved muscovite is likely due to terminal 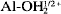 or Al-OH1/2− groups on these crystalline surfaces, which favor adsorption of Pb2+ ions and the subsequent nucleation and precipitation reactions. We also investigated the effect of drying rate on the morphology of the surface precipitates. Fast drying resulted in a nearly complete covered surface with a leaflike morphology, whereas slow drying resulted in more isolated surface clusters.
or Al-OH1/2− groups on these crystalline surfaces, which favor adsorption of Pb2+ ions and the subsequent nucleation and precipitation reactions. We also investigated the effect of drying rate on the morphology of the surface precipitates. Fast drying resulted in a nearly complete covered surface with a leaflike morphology, whereas slow drying resulted in more isolated surface clusters.
- Type
- Research Article
- Information
- Copyright
- Copyright © 1998, The Clay Minerals Society
References
- 13
- Cited by




