Article contents
Oxygen-Isotope Fractionation between Aluminum-Hydroxide Phases and Water at <60°C: Results of Decade-Long Synthesis Experiments
Published online by Cambridge University Press: 28 February 2024
Abstract
Oxygen-isotope data were obtained for synthetic aluminum-hydroxide phases precipitated over 65–125 mo and have been compared to results from similar experiments conducted for 3–56 mo. The Al(OH)3 polymorphs, gibbsite, nordstrandite, and bayerite, were synthesized, but gibbsite was dominant in most samples, and commonly the only phase present. Using pure gibbsite samples, the following oxygen-isotope fractionation factors, αgibbsite−H2O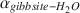 , were obtained: 1.0167 ± 0.0003 (9 ± 1°C), 1.0147 ± 0.0007 (24 ± 2°C), 1.0120 ± 0.0003 (51 ± 2°C). These values, and the associated equation for an oxygen-isotope geothermometer for the interval 0–60°C 103ln αgibbsite−H2O=2.04×106/T2−3.61×103/T+3.65
, were obtained: 1.0167 ± 0.0003 (9 ± 1°C), 1.0147 ± 0.0007 (24 ± 2°C), 1.0120 ± 0.0003 (51 ± 2°C). These values, and the associated equation for an oxygen-isotope geothermometer for the interval 0–60°C 103ln αgibbsite−H2O=2.04×106/T2−3.61×103/T+3.65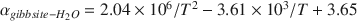 (T in K), are not significantly different from those obtained from experiments of much shorter duration. These results, and the good agreement with αgibbsite−H2O
(T in K), are not significantly different from those obtained from experiments of much shorter duration. These results, and the good agreement with αgibbsite−H2O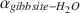 values obtained for well-constrained natural systems, suggest that the experimentally determined fractionation factors describe equilibrium conditions for gibbsite that has precipitated directly from solution.
values obtained for well-constrained natural systems, suggest that the experimentally determined fractionation factors describe equilibrium conditions for gibbsite that has precipitated directly from solution.
As also proposed by others using a modified-increment calculation, our synthesis experiments suggest that αAl(OH)3−H2O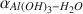 is polymorph-dependent at low temperatures and that a significant temperature-dependent trend exists in the values of αAl(OH)3−H2O
is polymorph-dependent at low temperatures and that a significant temperature-dependent trend exists in the values of αAl(OH)3−H2O . However, previously calculated fractionation factors obtained using the modified-increment method are higher than those obtained from the experiments, with this discrepancy becoming larger as temperature decreases.
. However, previously calculated fractionation factors obtained using the modified-increment method are higher than those obtained from the experiments, with this discrepancy becoming larger as temperature decreases.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2000, The Clay Minerals Society
References
- 8
- Cited by




