Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Garrido-Ramírez, E.G.
Theng, B.K.G
and
Mora, M.L.
2010.
Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions — A review.
Applied Clay Science,
Vol. 47,
Issue. 3-4,
p.
182.
Yuan, Guodong
2010.
Nanotechnologies for the Life Sciences.
Kaufhold, S.
Stanjek, H.
Penner, D.
and
Dohrmann, R.
2011.
The acidity of surface groups of dioctahedral smectites.
Clay Minerals,
Vol. 46,
Issue. 4,
p.
583.
Calabi-Floody, Marcela
Bendall, James S.
Jara, Alejandra A.
Welland, Mark E.
Theng, Benny K.G.
Rumpel, Cornelia
and
Mora, María de la Luz
2011.
Nanoclays from an Andisol: Extraction, properties and carbon stabilization.
Geoderma,
Vol. 161,
Issue. 3-4,
p.
159.
Fuchs, Marco
Abidin, Zaenal
Kübel, Christian
Weidler, Peter G.
Matsue, Naoto
Henmi, Teruo
Köster, Rainer
and
Gliemann, Hartmut
2012.
Patterned Deposition of Allophane Nanoparticles on Silicon Substrates.
Clays and Clay Minerals,
Vol. 60,
Issue. 5,
p.
456.
Garrido-Ramirez, Elizabeth G.
Sivaiah, Matte V.
Barrault, Joël
Valange, Sabine
Theng, Benny K.G.
Ureta-Zañartu, Maria Soledad
and
Mora, María de la Luz
2012.
Catalytic wet peroxide oxidation of phenol over iron or copper oxide-supported allophane clay materials: Influence of catalyst SiO2/Al2O3 ratio.
Microporous and Mesoporous Materials,
Vol. 162,
Issue. ,
p.
189.
Levard, C.
Doelsch, E.
Basile-Doelsch, I.
Abidin, Z.
Miche, H.
Masion, A.
Rose, J.
Borschneck, D.
and
Bottero, J.-Y.
2012.
Structure and distribution of allophanes, imogolite and proto-imogolite in volcanic soils.
Geoderma,
Vol. 183-184,
Issue. ,
p.
100.
Bishop, Janice L.
Rampe, Elizabeth B.
Bish, David L.
Abidin, Zaenal
Baker, Leslie L.
Matsue, Naoto
and
Henmi, Teruo
2013.
Spectral and Hydration Properties of Allophane and Imogolite.
Clays and Clay Minerals,
Vol. 61,
Issue. 1,
p.
57.
Cervini-Silva, Javiera
Antonio-Nieto-Camacho
Gomez-Vidales, Virginia
Ramirez-Apan, María Teresa
Palacios, Eduardo
Montoya, Ascención
Kaufhold, Stephan
Abidin, Zeanal
and
Theng, Benny K.G.
2014.
Lipid peroxidation and cytotoxicity induced by respirable volcanic ash.
Journal of Hazardous Materials,
Vol. 274,
Issue. ,
p.
237.
Wen, Y.L.
Xiao, J.
Li, H.
Shen, Q.R.
Ran, W.
Zhou, Q.S.
Yu, G.H.
and
He, X.H.
2014.
Long‐Term Fertilization Practices Alter Aluminum Fractions and Coordinate State in Soil Colloids.
Soil Science Society of America Journal,
Vol. 78,
Issue. 6,
p.
2083.
Medina, Jorge
Monreal, Carlos
Barea, José Miguel
Arriagada, César
Borie, Fernando
and
Cornejo, Pablo
2015.
Crop residue stabilization and application to agricultural and degraded soils: A review.
Waste Management,
Vol. 42,
Issue. ,
p.
41.
Filimonova, Svetlana
Kaufhold, Stephan
Wagner, Friedrich E.
Häusler, Werner
and
Kögel-Knabner, Ingrid
2016.
The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol.
Geochimica et Cosmochimica Acta,
Vol. 180,
Issue. ,
p.
284.
Kaufhold, Stephan
Kaufhold, Annette
and
Dohrmann, Reiner
2018.
Comparison of the Critical Coagulation Concentrations of Allophane and Smectites.
Colloids and Interfaces,
Vol. 2,
Issue. 1,
p.
12.
Wang, Shun
Du, Peixin
Yuan, Peng
Zhong, Xuemin
Liu, Yaqi
Liu, Dong
and
Deng, Liangliang
2018.
Changes in the structure and porosity of hollow spherical allophane under alkaline conditions.
Applied Clay Science,
Vol. 166,
Issue. ,
p.
242.
Sudadi, Untung
Anggriawan, Rendy
and
Anwar, Syaiful
2020.
Aplikasi kleinano dari tuf volkan Gunung Salak Indonesia sebagai adsorben alami kontaminan anionik: Fosfat perairan.
Jurnal Pengelolaan Sumberdaya Alam dan Lingkungan (Journal of Natural Resources and Environmental Management),
Vol. 9,
Issue. 4,
p.
1032.
Wang, Shun
Du, Peixin
Yuan, Peng
Liu, Yaqi
Song, Hongzhe
Zhou, Junming
Deng, Liangliang
and
Liu, Dong
2020.
Structural alterations of synthetic allophane under acidic conditions: Implications for understanding the acidification of allophanic Andosols.
Geoderma,
Vol. 376,
Issue. ,
p.
114561.
Bollinger, David L.
Erickson, Jessica
Stone-Weiss, Nicholas
Lere-Adams, Arumala Josiah
Karcher, Sam
Akin, Idil Deniz
and
McCloy, John S.
2021.
Structure of amorphous aluminosilicates obtained from mineral transformation: Potential path for partial remediation of alkaline bauxite residues.
Environmental Advances,
Vol. 6,
Issue. ,
p.
100136.
Anda, M
Kasno, A
Ginting, C B
Barus, P A
and
Purwanto, S
2021.
Response of Andisols to intensive agricultural land use: Implication on changes in P accumulation and colloidal surface charge.
IOP Conference Series: Earth and Environmental Science,
Vol. 648,
Issue. 1,
p.
012016.
Beram, Abdullah
2023.
Enhancing Surface Characteristics and Combustion Behavior of Black Poplar Wood through Varied Impregnation Techniques.
Applied Sciences,
Vol. 13,
Issue. 20,
p.
11482.
Baldermann, Andre
Stamm, Franziska M.
Farkaš, Juraj
Löhr, Stefan
Ratz, Bettina
Letofsky-Papst, Ilse
and
Dietzel, Martin
2024.
Precipitation of short-range order hydroxy aluminosilicate (HAS) and hydrous ferric silicate (HFS) at ambient temperature: Insights into mineral formation pathways, crystal chemistry and solubility-stability relationships.
Chemical Geology,
Vol. 646,
Issue. ,
p.
121911.
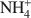 was 15.1 cmolc kg−1, but CH3NH3+${\rm{C}}{{\rm{H}}_3}{\rm{NH}}_3^ + $
was 15.1 cmolc kg−1, but CH3NH3+${\rm{C}}{{\rm{H}}_3}{\rm{NH}}_3^ + $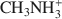 (mean diameter = 0.38 nm) only extracted 7.9 cmolc kg−1 of Ca2+. After 10 mM NaOH treatment (0.25 g:100 mL) of the allophane, the Ca2+ extracted by NH4+${\rm{NH}}_4^ + $
(mean diameter = 0.38 nm) only extracted 7.9 cmolc kg−1 of Ca2+. After 10 mM NaOH treatment (0.25 g:100 mL) of the allophane, the Ca2+ extracted by NH4+${\rm{NH}}_4^ + $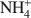 was 29.7 cmolc kg−1, 29.6 cmolc kg−1 by CH3NH3+${\rm{C}}{{\rm{H}}_3}{\rm{NH}}_3^ + $
was 29.7 cmolc kg−1, 29.6 cmolc kg−1 by CH3NH3+${\rm{C}}{{\rm{H}}_3}{\rm{NH}}_3^ + $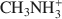 , and 29.4 cmolc kg−1 by (CH3)2NH2+${{\rm{(C}}{{\rm{H}}_3}{\rm{)}}_2}{\rm{NH}}_2^ + $
, and 29.4 cmolc kg−1 by (CH3)2NH2+${{\rm{(C}}{{\rm{H}}_3}{\rm{)}}_2}{\rm{NH}}_2^ + $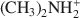 . The extraction of Ca2+ by the large C2H5NH3+${{\rm{C}}_2}{{\rm{H}}_5}{\rm{NH}}_3^ + $
. The extraction of Ca2+ by the large C2H5NH3+${{\rm{C}}_2}{{\rm{H}}_5}{\rm{NH}}_3^ + $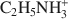 cation (mean diameter = 0.46 nm) only decreased to 26.1 cmolc kg−1, indicating that pore diameters were enlarged from ∼0.35 to 0.45 nm. The significant increase in Ca2+ retention after NaOH treatment was attributed to the dissociation of increased numbers of newly exposed silanol groups in the enlarged pores. The low Si/Al ratio of the NaOH-dissolved material (0.35) and the decreased intensity of the 348 cm−1 IR band also suggested selective dissolution of the pore region. For allophane with a high Si/Al ratio (0.99) and much accessory polymeric Si, dissolution of polymeric Si and of the pore region occurred simultaneously. Alkali treatment produced a smaller increase in pore size and Ca2+ retention for allophanes with large Si/Al ratios than for allophanes with small Si/Al ratios. It was concluded that by altering the dilute alkali treatment conditions and varying the Si/Al ratio of allophane, the extent of structural modification or pore enlargement of the hollow spheres might be controlled.
cation (mean diameter = 0.46 nm) only decreased to 26.1 cmolc kg−1, indicating that pore diameters were enlarged from ∼0.35 to 0.45 nm. The significant increase in Ca2+ retention after NaOH treatment was attributed to the dissociation of increased numbers of newly exposed silanol groups in the enlarged pores. The low Si/Al ratio of the NaOH-dissolved material (0.35) and the decreased intensity of the 348 cm−1 IR band also suggested selective dissolution of the pore region. For allophane with a high Si/Al ratio (0.99) and much accessory polymeric Si, dissolution of polymeric Si and of the pore region occurred simultaneously. Alkali treatment produced a smaller increase in pore size and Ca2+ retention for allophanes with large Si/Al ratios than for allophanes with small Si/Al ratios. It was concluded that by altering the dilute alkali treatment conditions and varying the Si/Al ratio of allophane, the extent of structural modification or pore enlargement of the hollow spheres might be controlled.

