Article contents
Eu incorporation behavior of a Mg-Al-Cl layered double hydroxide
Published online by Cambridge University Press: 01 January 2024
Abstract
From leaching experiments with metallic uranium-aluminum research reactor fuel elements in repository-relevant MgCl2-rich salt brines, a Mg-Al layered double hydroxide (LDH) with chloride as the interlayer anion was identified as a crystalline secondary phase component. The incorporation behavior of europium into the structure of the Mg-Al-Cl LDH was investigated. Synthesis via co-precipitation was performed. The Mg-Al-Eu-Cl LDH obtained was treated with a concentrated ammonium carbonate solution. No release of Eu was detected; hence the molar stoichiometry of the LDH remained stable with respect to Mg, Al and Eu. This chemical behavior might be the first indication of the incorporation of Eu.
The material was further examined by powder X-ray diffraction. Structural parameters were obtained from comparisons of simulated and experimental diffraction patterns of a CO32−${\rm{CO}}_3^{2 - }$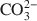 -exchanged Mg-Al-Eu LDH and a Mg-Al LDH. The two materials showed different behaviors according to stacking order and lattice parameters. This is an indirect indication of the incorporation of Eu.
-exchanged Mg-Al-Eu LDH and a Mg-Al LDH. The two materials showed different behaviors according to stacking order and lattice parameters. This is an indirect indication of the incorporation of Eu.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2007, The Clay Minerals Society
References
- 10
- Cited by




