Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Ramírez-Valle, V.
Jiménez de Haro, M. C.
Avilés, M. A.
Pérez-Maqueda, L. A.
Durán, A.
Pascual, J.
and
Pérez-Rodríguez, J. L.
2006.
Effect of interlayer cations on high-temperature phases of vermiculite.
Journal of Thermal Analysis and Calorimetry,
Vol. 84,
Issue. 1,
p.
147.
Pérez-Rodríguez, J.L.
Wiewióra, A.
Drapala, J.
and
Pérez-Maqueda, L.A.
2006.
The effect of sonication on dioctahedral and trioctahedral micas.
Ultrasonics Sonochemistry,
Vol. 13,
Issue. 1,
p.
61.
Balek, V.
Pérez-Rodríguez, J. L.
Pérez-Maqueda, L. A.
Šubrt, J.
and
Poyato, J.
2007.
Thermal behaviour of ground vermiculite.
Journal of Thermal Analysis and Calorimetry,
Vol. 88,
Issue. 3,
p.
819.
Burzo, E.
2007.
Phyllosilicates.
p.
366.
Balek, V.
Subrt, J.
Pérez-Maqueda, L.A.
Benes, M.
Bountseva, I.M.
Beckman, I.N.
and
Pérez-Rodríguez, J.L.
2008.
Thermal behavior of ground talc mineral.
Journal of Mining and Metallurgy, Section B: Metallurgy,
Vol. 44,
Issue. 1,
p.
7.
Balek, V.
Beneŝ, M.
Ŝubrt, J.
Pérez-Rodriguez, J. L.
Sánchez-Jiménez, P. E.
Pérez-Maqueda, L. A.
and
Pascual-Cosp, J.
2008.
Thermal characterization of montmorillonite clays saturated with various cations.
Journal of Thermal Analysis and Calorimetry,
Vol. 92,
Issue. 1,
p.
191.
Duman, Osman
and
Tunç, Sibel
2008.
Electrokinetic Properties of Vermiculite and Expanded Vermiculite: Effects of pH, Clay Concentration and Mono- and Multivalent Electrolytes.
Separation Science and Technology,
Vol. 43,
Issue. 14,
p.
3755.
de Farias, Robson Fernandes
2009.
Chemistry on Modified Oxide and Phosphate Surfaces - Fundamentals and Applications.
Vol. 17,
Issue. ,
p.
113.
Alves, A. P. M.
Araujo, A. S.
Bezerra, F. A.
Sousa, K. S.
Lima, S. J. G.
and
Fonseca, M. G.
2014.
Kinetics of dehydration and textural characterizations of selectively leached vermiculites.
Journal of Thermal Analysis and Calorimetry,
Vol. 117,
Issue. 1,
p.
19.
Balek, Vladimir
2015.
Encyclopedia of Surface and Colloid Science, Third Edition.
p.
2486.
Duman, Osman
Tunç, Sibel
and
Polat, Tülin Gürkan
2015.
Determination of adsorptive properties of expanded vermiculite for the removal of C. I. Basic Red 9 from aqueous solution: Kinetic, isotherm and thermodynamic studies.
Applied Clay Science,
Vol. 109-110,
Issue. ,
p.
22.
Ma, Lingya
Su, Xiaoli
Xi, Yunfei
Wei, Jingming
Liang, Xiaoliang
Zhu, Jianxi
and
He, Hongping
2019.
The structural change of vermiculite during dehydration processes: A real-time in-situ XRD method.
Applied Clay Science,
Vol. 183,
Issue. ,
p.
105332.
Liu, Hanqing
Sun, Keyan
Shi, Xiaoyu
Yang, Huning
Dong, Hongsheng
Kou, Yan
Das, Pratteek
Wu, Zhong-Shuai
and
Shi, Quan
2021.
Two-dimensional materials and their derivatives for high performance phase change materials: emerging trends and challenges.
Energy Storage Materials,
Vol. 42,
Issue. ,
p.
845.
Bobrowski, Artur
Kaczmarska, Karolina
Sitarz, Maciej
Drożyński, Dariusz
Leśniak, Magdalena
Grabowska, Beata
and
Nowak, Daniel
2021.
Dehydroxylation of Perlite and Vermiculite: Impact on Improving the Knock-Out Properties of Moulding and Core Sand with an Inorganic Binder.
Materials,
Vol. 14,
Issue. 11,
p.
2946.
Lv, Xifeng
Fan, Chaoqun
Han, Ying
Tang, Xiaojin
Zhang, Changwei
Cai, Di
and
Chen, Huidong
2023.
Expanded Vermiculite/D-Mannitol as Shape-Stable Phase Change Material for Medium Temperature Heat Storage.
Materials,
Vol. 16,
Issue. 18,
p.
6101.
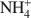 -exchanged vermiculite samples. In addition, vermiculite samples subjected to a chemical treatment with an aqueous solution of (NH4)2SiF6 and partially or totally re-saturated with Na+ ions were also investigated by ETA. The ETA results of natural Mg2+-vermiculite, Na+-vermiculite and NH4+
-exchanged vermiculite samples. In addition, vermiculite samples subjected to a chemical treatment with an aqueous solution of (NH4)2SiF6 and partially or totally re-saturated with Na+ ions were also investigated by ETA. The ETA results of natural Mg2+-vermiculite, Na+-vermiculite and NH4+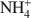 -vermiculite gave supplementary information about microstructure changes of the samples observed under dynamic heating conditions. The method has proved to be very useful for characterization of microstructure changes due to modification in the interlayer space of samples during the heat treatment. The crystallization of vermiculite into new phases, such as enstatite (for NH4+
-vermiculite gave supplementary information about microstructure changes of the samples observed under dynamic heating conditions. The method has proved to be very useful for characterization of microstructure changes due to modification in the interlayer space of samples during the heat treatment. The crystallization of vermiculite into new phases, such as enstatite (for NH4+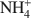 -vermiculite and Mg2+-vermiculite) and forsterite (for Na+-vermiculite) was also observed by ETA.
-vermiculite and Mg2+-vermiculite) and forsterite (for Na+-vermiculite) was also observed by ETA.



