Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Fialips, Claire-Isabelle
Petit, Sabine
Decarreau, Alain
and
Beaufort, Daniel
2000.
Influence of Synthesis pH on Kaolinite “Crystallinity” and Surface Properties.
Clays and Clay Minerals,
Vol. 48,
Issue. 2,
p.
173.
Jankovič, Ľuboš
and
Komadel, Peter
2000.
Catalytic Properties of a Heated Ammonium-Saturated Dioctahedral Smectite.
Collection of Czechoslovak Chemical Communications,
Vol. 65,
Issue. 9,
p.
1527.
Joussein, Emmanuel
Petit, Sabine
and
Decarreau, Alain
2001.
Une nouvelle méthode de dosage des minéraux argileux en mélange par spectroscopie IR.
Comptes Rendus de l'Académie des Sciences - Series IIA - Earth and Planetary Science,
Vol. 332,
Issue. 2,
p.
83.
Madejová, Jana
and
Komadel, Peter
2001.
Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods.
Clays and Clay Minerals,
Vol. 49,
Issue. 5,
p.
410.
Jozefaciuk, G
Muranyi, A
and
Alekseeva, T
2002.
Effect of extreme acid and alkali treatment on soil variable charge.
Geoderma,
Vol. 109,
Issue. 3-4,
p.
225.
Bishop, J.L
Banin, A
Mancinelli, R.L
and
Klovstad, M.R
2002.
Detection of soluble and fixed NH4+ in clay minerals by DTA and IR reflectance spectroscopy: a potential tool for planetary surface exploration.
Planetary and Space Science,
Vol. 50,
Issue. 1,
p.
11.
Jozefaciuk, Grzegorz
2002.
Effect of Acid and Alkali Treatments on Surface-Charge Properties of Selected Minerals.
Clays and Clay Minerals,
Vol. 50,
Issue. 5,
p.
647.
Cuevas, Jaime
de la Villa, Raquel Vigil
Ramirez, Susana
Petit, Sabine
Meunier, Alain
and
Leguey, Santiago
2003.
Chemistry of Mg Smectites in Lacustrine Sediments from the Vicalvaro Sepiolite Deposit, Madrid Neogene Basin (Spain).
Clays and Clay Minerals,
Vol. 51,
Issue. 4,
p.
457.
Boonmung, Suwanee
and
Riley, Mark R.
2003.
Quantitative Analysis of Added Ammonium and Nitrate in Silica Sand and Soil Using Diffuse Reflectance Infrared Spectroscopy.
Spectroscopy Letters,
Vol. 36,
Issue. 3,
p.
251.
Pironon, J .
Pelletier, M.
De Donato, P.
and
Mosser-Ruck, R.
2003.
Characterization of smectite and illite by FTIR spectroscopy of interlayer NH4+ cations.
Clay Minerals,
Vol. 38,
Issue. 2,
p.
201.
Jiménez de Haro, M.C.
Pérez-Rodríguez, J.L.
Poyato, J.
Pérez-Maqueda, L.A.
Ramírez-Valle, V.
Justo, A.
Lerf, A.
and
Wagner, F.E.
2005.
Effect of ultrasound on preparation of porous materials from vermiculite.
Applied Clay Science,
Vol. 30,
Issue. 1,
p.
11.
Dontsova, Katerina M.
Norton, L. Darrell
and
Johnston, Cliff T.
2005.
Calcium and Magnesium Effects on Ammonia Adsorption by Soil Clays.
Soil Science Society of America Journal,
Vol. 69,
Issue. 4,
p.
1225.
Petit, S.
2006.
Handbook of Clay Science.
Vol. 1,
Issue. ,
p.
909.
Carrado, K.A.
Decarreau, A.
Petit, S.
Bergaya, F.
and
Lagaly, G.
2006.
Handbook of Clay Science.
Vol. 1,
Issue. ,
p.
115.
Petit, S.
Righi, D.
and
Madejová, J.
2006.
Infrared spectroscopy of NH4+-bearing and saturated clay minerals: A review of the study of layer charge.
Applied Clay Science,
Vol. 34,
Issue. 1-4,
p.
22.
Burzo, E.
2007.
Phyllosilicates.
p.
366.
Šucha, Vladimír
Uhlík, Peter
Madejová, Jana
Petit, Sabine
Kraus, Ivan
and
Puškelová, L’ubica
2007.
Particle properties of hydrothermal ammonium-bearing illite-smectite.
Clays and Clay Minerals,
Vol. 55,
Issue. 1,
p.
36.
Gautier, M.
Muller, F.
Beny, J.-M.
Le Forestier, L.
Alberic, P.
and
Baillif, P.
2009.
Interactions of ammonium smectite with low-molecular-weight carboxylic acids.
Clay Minerals,
Vol. 44,
Issue. 2,
p.
207.
Li, Zhenze
Tang, Xiaowu
Chen, Yunmin
and
Wang, Yan
2009.
Sorption Behavior and Mechanism of Pb(II) on Chinese Loess.
Journal of Environmental Engineering,
Vol. 135,
Issue. 1,
p.
58.
Dali-Youcef, Z
Queneudec, M
and
Dheilly, R M
2010.
Study of the retention of organic pollutants starting from aqueous solutions by adsorption on local clays.
IOP Conference Series: Materials Science and Engineering,
Vol. 13,
Issue. ,
p.
012029.
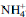 spectrum of the
spectrum of the 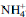 -clays: application to the evaluation of the layer charge
-clays: application to the evaluation of the layer charge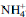 -saturated smectites were examined in terms of their charge characteristics. The υ4
-saturated smectites were examined in terms of their charge characteristics. The υ4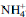 band near 1440 cm-1, observed in the DRIFTS spectra (obtained without use of a KBr matrix), was assigned to the vibrations of
band near 1440 cm-1, observed in the DRIFTS spectra (obtained without use of a KBr matrix), was assigned to the vibrations of 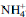 ions compensating the negative charge of the clays. When KBr was used as a diluting matrix, the υ4
ions compensating the negative charge of the clays. When KBr was used as a diluting matrix, the υ4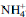 band was located at 1400 and/or 1440 cm-1. The band at 1400 cm-1, related to NH4Br, originated from the replacement of
band was located at 1400 and/or 1440 cm-1. The band at 1400 cm-1, related to NH4Br, originated from the replacement of 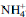 in the clay by K+ from the KBr. For swelling clay minerals this band indicates that layers have permanent low charge density and/or variable charge. For non-swelling clay minerals, the 1400 cm-1 band characterizes the presence of variable charges only. The υ4
in the clay by K+ from the KBr. For swelling clay minerals this band indicates that layers have permanent low charge density and/or variable charge. For non-swelling clay minerals, the 1400 cm-1 band characterizes the presence of variable charges only. The υ4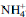 band at 1440 cm-1 suggests that
band at 1440 cm-1 suggests that 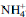 in the clay was not replaced by K+ from KBr and remains in the interlayer space of the clay minerals. This absorption is due to
in the clay was not replaced by K+ from KBr and remains in the interlayer space of the clay minerals. This absorption is due to 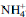 compensating only permanent charge in the interlayers, or part of the interlayers with a high charge density. The presence of both bands at 1400 cm-1 and 1440 cm-1 in the IR spectrum suggests that the clays studied have a heterogeneous interlayer charge.
compensating only permanent charge in the interlayers, or part of the interlayers with a high charge density. The presence of both bands at 1400 cm-1 and 1440 cm-1 in the IR spectrum suggests that the clays studied have a heterogeneous interlayer charge.



