Article contents
Effect of amino acid (glycine) on the distribution of transition metal (Co-Ni-Zn-Cu) and magnesium divalent ions between silicate gels and aqueous solutions: an experimental study
Published online by Cambridge University Press: 09 July 2018
Abstarct
The study of M and Mg ion exchange (M = Co, Ni, Cu, Zn), between silicate gels SiO2.q(M, Mg)O.nH2O and amino acid (glycine) saline aqueous solutions (M, Mg)SO4, shows that the introduction of the complexing agent completely upsets the original distribution equilibria of M and Mg between the two types of phases. The values of the measured bulk distribution coefficient
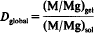
are lowered considerably relative to the inorganic reference distribution coefficients. The lowering of D may be accounted for by calculating the contents of the different species, MA, MA+, M2+, under which the metallic element M is present in the solutions. The values of
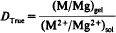
resulting from the calculated M2+ contents are identical to the values of Dref, determined in systems without a complexing agent.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1988
References
- 1
- Cited by




