There is a paucity of evidence-based literature on most aspects of late-presenting CHD. Similarly, guidelines on operability in infants and children with late CHD are scarce. This article reviews available published information and common practices of cardiologists, surgeons, and intensivists from across the globe who routinely manage late-presenting CHD. Units with a large case load of late CHD are typically those that can least afford expensive rescue strategies, and thus most practices are based on less expensive therapies. Finally, the management of late CHD will remain an evolving story as more data and experience accumulate.
Background
Congenital cardiac abnormalities are the most common of all birth defects and are responsible for the majority of deaths due to congenital abnormalities. It is estimated that, worldwide, approximately one million children are born annually with CHD. Of these, the majority are born in low- and middle-income countries where, regardless of the optimal strategy, surgery in early infancy is not always feasible.Reference Nguyen, Leon-Wyss, Iyer and Pezzela 1
With improving economic conditions and increasing availability of paediatric cardiac care, many infants, older children, and adults who would previously not have had access to these services present for cardiac care for the first time relatively late in their natural history, with increased risk of perioperative morbidity and mortality, or even inoperability. The challenge lies in eliminating late CHD as a risk factor for early mortality and minimising morbidity using cost-effective strategies. With reduction in early mortality, there has been a shift towards treatment modalities that are also associated with better long-term quality of life.
In this article, potential strategies and guidelines for the management of late-presenting CHD will be discussed. This is a broad topic with limited supportive literature. The recommendations are therefore based upon the available published literature, as well as opinions of surgeons, cardiologists, and other care-givers from low- and middle-income countries who deal with children presenting with late CHD. The focus of this review is on commonly encountered clinical scenarios that pose difficulties in decision making.
What is “late-presenting CHD” and why does it occur?
A detailed literature search does not yield a clear definition of late CHD. Most surgeons, cardiologists, and intensivists would concur that late CHD should not be confused with adult CHD and has no specific relation to age at presentation. Instead it would appear that late CHD refers to presentation late in the natural history of the specific cardiac defect, with consequent transient or irreversible haemodynamic and pathologic alterations that would impact the medical and surgical approach, risk, and outcome. Thus, a 10-year-old child presenting with a moderate-sized atrial septal defect may not be considered late, but a newborn with transposition of great arteries and intact ventricular septum presenting at 1 month of age with cardiovascular collapse would be considered to be a late presentation. The late presentation of CHD occurs owing to a variety of reasons: late diagnosis and referral; limited resources and infrastructure, or geographic factors precluding timely diagnosis and transfer to a cardiac centre; and low levels of awareness and inappropriate medical advice. Some or all of these factors are routinely encountered in low- and middle-income countries; hence, late presentation is common.Reference Nguyen, Leon-Wyss, Iyer and Pezzela 1 – Reference Rahajoe 4
Consequences of late-presenting CHD
The physiological and pathological consequences of late presentation vary according to the type of lesion and associated circulatory abnormalities and may be broadly grouped as follows:
-
∙ Newborns with critical, duct-dependent CHD including coarctation of the aorta or pulmonary atresia, or those with CHD dependent on intracardiac mixing such as transposition of great arteries, typically present with circulatory collapse, and varying degrees of multiorgan dysfunction adversely impacting surgical outcomes.Reference Brown, Ridout, Hoskote, Verhulst, Ricci and Bull 5 , Reference Bonnet, Coltri and Butera 6
-
∙ Older infants and children with chronic and relatively stable CHD associated with left-to-right shunts and pulmonary over-circulation, including large ventricular septal defects, double-outlet right ventricle with ventricular septal defect, or truncus arteriosus, may present with other consequences. These include frequent respiratory infections, cachexia, and pulmonary hypertension.
-
∙ Chronic cyanosis in the setting of unoperated tetralogy of Fallot, single ventricle with pulmonary obstruction, or Eisenmengers syndrome can lead to polycythaemia with or without disseminated intravascular coagulopathy, hepatic or renal dysfunction, or stroke.
-
∙ Unoperated valvular disease or indeed any intracardiac defect, particularly in the setting of poor nutrition and adverse environmental factors, can significantly increase the risk of endocarditis. Presentation may be with acute circulatory collapse due to acute onset of severe valvular regurgitation or with manifestations of pulmonary or systemic embolisation. Neurologic complications from brain abscesses are also not uncommon.
Late-presenting CHD associated with left-to-right shunts
Left-to-right shunts including atrial septal defects, ventricular septal defects, and patent ductus arteriosus are among the most common forms of CHD that present late, resulting in varying degrees of pulmonary hypertension. With timely surgery, mortality and morbidity for these defects is very low. The challenge lies in deciding operability and, if operable, ensuring good-quality long-term survival.
There is little consensus as to the definition of operability in terms of echocardiographic data or catheterisation-derived pulmonary vascular resistance index measurements.Reference Lopes, Barst and Haworth 7 – Reference Myers, Tissot and Beghetti 11 Similarly, there is wide variability in the surgical approach. In general, current recommendations are relatively conservative – a pulmonary vascular resistance index cut-off of <6 Wood units m2 Reference Lopes, Barst and Haworth 7 (Table 1). However, some institutions have offered surgery for patients with a pulmonary vascular resistance index up to 15 Wood units/m2.Reference Novik, Gurbuz and Watson 12 – Reference Sridhar, Sahayaraj and Lakshmi 16 Surgical strategies to overcome perioperative pulmonary hypertensive crises have varied from the use of a decompressive atrial communication to valved patch closure of a ventricular septal defect or atrial septal defect (Table 2), or pulmonary artery banding with excellent early survival.Reference Novik, Gurbuz and Watson 12 – Reference Oliver, Gallego and González 19 Talwar et alReference Talwar, Keshri and Choudhary 15 reported excellent early and midterm outcomes with unidirectional valved patch with regression of pulmonary hypertension in the midterm; however, there have also been reports of worsening pulmonary hypertension in the longer term.Reference Sridhar, Sahayaraj and Lakshmi 16 It would appear that a standardised approach to early assessment and close postoperative follow-up would ensure that surgery is offered appropriately and not to patients with established pulmonary vascular disease, thereby offering the best possible outcomes for these high-risk patients.Reference Myers, Tissot and Beghetti 11 , Reference Beghetti, Galie and Bonnet 20 – Reference Dimopoulos, Wort and Gatzoulis 22
Table 1 Preoperative evaluation of paediatric patients with congenital systemic-to-pulmonary shunts – findings that may indicate a favourable or unfavourable response to correction of the cardiac defect.
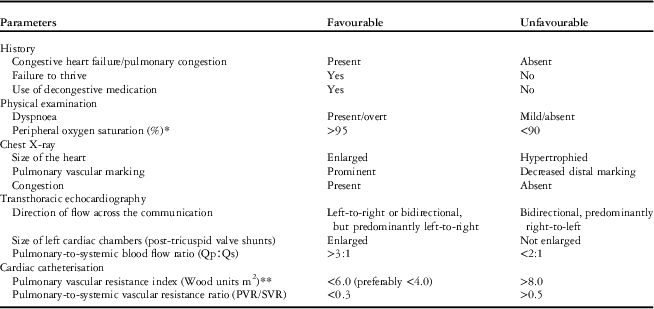
PVR=pulmonary vascular resistance; SVR=systemic vascular resistance
Modified from Lopes et al, “Guidelines and Consensus. Repair of congenital heart disease with associated pulmonary hypertension in children: what are the minimal investigative procedures?”7.
* Basal saturations of 90–95% should also be reviewed case-by-case
** Pulmonary vascular resistance index 4–8 should be individualised or managed case-by-case
Table 2 Summary of results with different surgical procedures in ventricular septal defects and severe pulmonary hypertension.
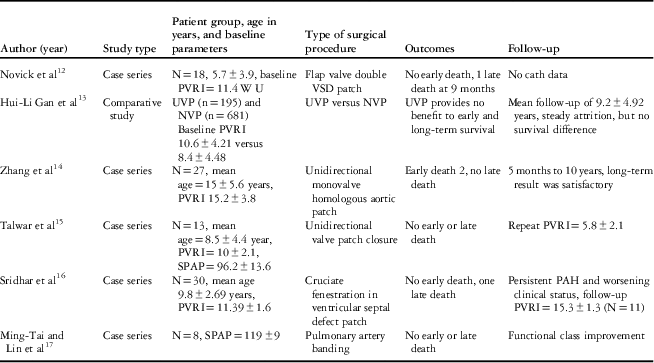
NVP=non-valve patch; PAH=Pulmonary artery hypertension; PVRI=pulmonary vascular resistance index; SPAP=systolic pulmonary artery pressure; UVP=unidirectional valve patch; VSD=ventricular septal defect; WU=wood unit
More complex cardiac malformations such as double-outlet right ventricle, transposition of great arteries with ventricular septal defect, or truncus arteriosus presenting late pose even greater challenges in deciding surgical candidacy. Current consensus for these conditions is that clinical criteria such as baseline oxygen saturation and responsiveness to supplemental oxygen, as well as radiologic criteria suggestive of pulmonary over-circulation, may help to define operability.Reference Bajpai, Shah, Misri, Rao, Suresh and Maheshwari 23
Late-presenting tetralogy of Fallot and associated physiology
Tetralogy of Fallot is the most common cyanotic heart defect outside the neonatal period. Nonetheless, an extensive literature search yielded only case reports of the polycythaemic hypercyanotic tetralogy of Fallot, with search terms such as late tetralogy of Fallot, hypercyanotic tetralogy of Fallot, bleeding and tetralogy of Fallot, intracranial bleeds and tetralogy of Fallot, endotracheal bleeding, and collaterals in tetralogy of Fallot. The following recommendations represent consensus opinion from multiple high-volume units across low- and middle-income countries that have significant experience in managing patients with tetralogy of Fallot with long-standing cyanosis and polycythaemia.
Complete repair during the first year of life is the surgical approach of choice for patients with tetralogy of Fallot in high-income countries. In low- and middle-income countries, however, patients with tetralogy of Fallot commonly present for the first time during childhood, adolescence, or even late adulthood.Reference Talwar, Meena and Choudhary 24 Some would be natural survivors of so-called “good anatomy tetralogy of Fallot” with minimal cyanosis and therefore manifest limited consequences of chronic cyanosis. Most patients, however, are markedly cyanotic and may manifest the deleterious sequelae of chronic polycythaemia with hyperviscosity and varying degrees of consumptive coagulopathy. In the absence of anatomic limitations such as significant hypoplasia of the branch pulmonary arteries, a definitive complete repair is the goal for all patients. A good surgical outcome in these patients is dependent on the following: detailed preoperative evaluation that dictates the specific preoperative preparation required; and careful customised cardiopulmonary bypass strategy, surgical approach, and perioperative care. We recommend the following.
Preoperative evaluation
-
∙ CT angiogram to assess pulmonary artery anatomy and delineate aortopulmonary collaterals, as these can significantly affect cerebral protection during surgery, and can predispose to major postoperative pulmonary bleeds. A decision to embolise these collaterals preoperatively is based on size and distribution.
-
∙ Detailed haematological work-up, with emphasis on coagulation profile, platelet count, and function. Profound polycythaemia secondary to long-standing cyanosis can lead to impaired coagulation and thrombocytopaenia.
-
∙ Detailed assessment of liver and kidney function.
-
∙ CT scan/MRI of the brain to exclude occult haemorrhage, recent or old infarcts, healed or active brain abscesses, and porencephaly.
Preoperative preparation
-
∙ Cardiac catheterisation: significant aortopulmonary collaterals should be occluded in the catheterisation laboratory, with the operating room in readiness for immediate surgery in the event of significant desaturation.
-
∙ Haematologic: isovolaemic phlebotomy should be considered in order to reduce the haematocrit to below 60%. The evidence for or against this practice is equivocal. Preoperative folic acid supplementation may improve thrombocytopaenia if surgery is not imminent. If there is persistent thrombocytopaenia and an unacceptable risk of bleeding associated with cardiopulmonary bypass, a staged approach using a modified Blalock–Taussig shunt in the first instance can be considered.
-
∙ Blood bank: adequate stocks of blood and blood products should be kept in readiness.
Intraoperative approach
-
∙ Nasal intubation or naso-gastric tubes should be avoided to prevent adenoidal haemorrhage.
-
∙ A higher-than-usual haematocrit is maintained on cardiopulmonary bypass to ensure adequate perfusion pressures.
-
∙ Normothermic or mildly hypothermic bypass is used to avoid further exacerbating impaired coagulation.
-
∙ Increased left atrial return, due to small collaterals, is managed with efficient suction rather than reducing pump flows.
-
∙ Excessive infundibular resection is avoided.Reference Kaushal, Radhakrishanan and Dagar 25 The transannular patch should be limited and some residual dynamic gradient may be desirable at the end of repair.Reference Kaushal, Radhakrishanan and Dagar 25
-
∙ Epicardial or transoesophageal echocardiography is used for assessing repair.
-
∙ Elective primary pulmonary valve replacement is often needed/preferred in adults to avoid free pulmonary regurgitation following complete repair.
-
∙ Meticulous haemostasis is achieved.
Postoperative care
Bleeding and a low cardiac output state are the problems most commonly encountered in the ICU. Postoperative bleeding, particularly pulmonary or intracranial, remains an important cause of mortality in this population. Although diastolic right ventricular dysfunction is common after tetralogy of Fallot repair, the postoperative course of late-presenting patients may also be complicated by systolic left ventricular dysfunction due to chronic hypoxaemia. The following anticipatory guidelines are recommeded:
-
∙ Avoidance of haemodilution should be a routine aspect of postoperative management to avoid low systemic vascular resistance and hypotension. We suggest that haematocrit is ideally maintained at 40–45%.
-
∙ Right ventricular diastolic dysfunction is to be anticipated. Measures to improve right ventricular function include maintaining adequate central venous pressure of 10–12 mmHg; ventilation with low mean airway pressures, or early extubation, unless there is a concern for reperfusion injury or pulmonary haemorrhage; and avoidance of β-adrenergic catecholamines. In terms of vasoactive support, a combination of milrinone and noradrenaline (norepinephrine) is considered effective.
-
∙ Mechanical ventilation must be individualised depending on the pulmonary status, particularly in the presence of endo-bronchial bleeding, and right and left ventricular function. Higher-than-usual levels of positive end expiratory pressure may sometimes be required to assist the lungs and left heart. It should be noted that positive end expiratory pressure titration should be very cautious, as the increase in mean airway pressure may increase right ventricle afterload, which already has severe diastolic dysfunction, and therefore worsen the haemodynamic state.
-
∙ Chronic polycythaemia increases the risk of postoperative bleeding from surgical sites, as well as internal organs. Patients should be closely monitored for signs of bleeding, and fresh frozen plasma, platelets, and coagulation factors should be used to optimise coagulation status in the presence of bleeding.
-
∙ Persistent endotracheal bleeding warrants urgent investigation. In the absence of coagulopathy, additional collaterals or a significant large additional or residual ventricular septal defect should be ruled out.
With these perioperative measures in place, discharge mortality can be reduced to <1%.
The late presentation of single-ventricle physiology with polycythaemia
This is a complex scenario, and a full discussion is beyond the scope of this article. The key consideration for these patients is candidacy for a primary Fontan operation. This decision requires careful multidisciplinary evaluation of systemic ventricular function, atrioventricular valve regurgitation, and collateral circulation. These patients are often profoundly cyanosed and their preoperative preparation is similar to that for the polycythaemic tetralogy of Fallot. During the postoperative phase, there is a tendency for protracted effusions to develop, particularly late-onset pericardial effusions requiring drainage. Early survival in most units is excellent and parallels the staged Fontan. However, owing to inadequate follow-up data, long-term outcomes remain uncertain.
The late-presenting newborn with critical CHD
Critical heart defects commonly missed at discharge from the maternity wards include transposition of great arteries with intact ventricular septum, coarctation of the aorta, and total anomalous pulmonary venous connection – all of which are treatable with low-risk corrective surgery.Reference Mellander 26 Approximately 40% of those discharged present with cardiovascular collapse, making subsequent management challenging, and significantly increasing perioperative morbidity and mortality.Reference Brown, Ridout, Hoskote, Verhulst, Ricci and Bull 5 , Reference Granelli, Wennergren and Sandberg 27
Before any intervention, the work-up after initial resuscitation and stabilisation of newborns presenting with critical CHD should include assessment of all organ systems and, where available, MRI of the brain to rule out injury related to hypoxaemia or hypoperfusion.Reference Petit, Rome and Wernovsky 28
The sick newborn with coarctation or near-total arch interruption often has severe biventricular dysfunction, as well as multiorgan dysfunction and often coexistent sepsis. Many units now would do a temporising catheter-based balloon dilatation of the coarctation, which usually results in relatively rapid recovery and >95% chance of discharge.Reference Bouzguenda, Marini, Ou, Boudjemline, Bonnet and Agnoletti 29 , Reference McGuinness, Elhassan and Lee 30 The infant is closely followed up and re-admitted for an elective surgical repair as and when there is recoarctation.
Transposition of the great arteries with intact ventricular septum
The sick neonate with transposition of great arteries and intact interventricular septum presenting with circulatory collapse and end-organ dysfunction usually has cardiovascular compromise owing to inadequate inter-circulatory mixing and/or associated Gram-negative or fungal sepsis – in 20–30% of cases. In these patients, stabilisation procedures may include a temporising high-risk balloon atrial septostomy or blade septectomy, if the septum is very thickened, interatrial stent, or patent ductus arteriosus stent.Reference Kothari, Ramakrishnan, Senguttuvan, Gupta and Bisoi 31 , Reference Sivakumar 32
The major challenge in decision making in these cases relates to the optimal timing of surgery and then the most appropriate initial operation in a patient with a potentially unprepared left ventricle. This decision is usually taken on a case-by-case basis. In Asia, where the problem of perioperative multi-resistant Gram-negative sepsis – a major risk factor for mortality – is significant,Reference Reddy, Kappanayil and Balachandran 33 many units are increasingly opting to treat the sepsis, discharge, and then review, rather than subjecting an infant with partially treated sepsis to cardiopulmonary bypass, and then having to manage fulminant sepsis in the postoperative period.
As far as we are aware, most surgeons in low- and middle-income settings would prefer to perform an arterial switch as the most physiologic procedure for all infants with transposition of great arteries with intact interventricular septum.Reference Nathan 34 – Reference Bisoi, Ahmed and Malankar 41 A primary arterial switch is offered regardless of age when the left ventricle is considered prepared or non-regressed. However, when the left ventricle is considered to be regressed, there is debate as to whether one should opt for a primary arterial switch or a two-stage switch after “rapid” left ventricle preparation with a pulmonary artery band. Several criteria for regression of the left ventricle in late-presenting transposition of great arteries have been proposed:
-
∙ Echocardiographic parameters: left ventricle shape – “banana shaped”; interventricular septum – moving with right ventricle rather than left ventricle; left ventricle posterior wall thickness – <3.0 mm; left ventricle mass <35 gm/m2; low left ventricle end diastolic volume; and non-quantitative assessment of the left ventricle.
-
∙ Haemodynamic parameters – left ventricle/right ventricle pressure ratio <0.7.
-
∙ Intraoperative response to trial of pulmonary artery banding.
Today, there is sufficient level 2 evidence to indicate that a primary arterial switch can be performed until 8 weeks of age with outcomes comparable to those of earlier repair.Reference Edwin, Mamorare, Brink and Kinsley 39 Most large-volume units in low- and middle-income settings would still prefer a delayed primary arterial switch in infants, even up to 3 months of age in a borderline regressed left ventricle, to a two-stage switch. The rapid two-stage switch is no longer popular even although one of the early series from India reported acceptable outcomes.Reference Iyer, Sharma and Kumar 42 This is because the first stage of the two-stage operation is often followed by a highly unpredictable and stormy ICU course. The relatively inexperienced medical and nursing staff of many of the ICUs in low- and middle-income settings prefer to manage the more predictable course of postoperative left ventricle failure following a late primary arterial switch (Table 3). There are no easy solutions, and the issue remains an evolving story as low- and middle-income countries witness steady improvements in intraoperative strategies, myocardial protection, and postoperative intensive care.
Table 3 Late primary arterial switch experience with mortality and mechanical circulatory support (MCS) requirement.
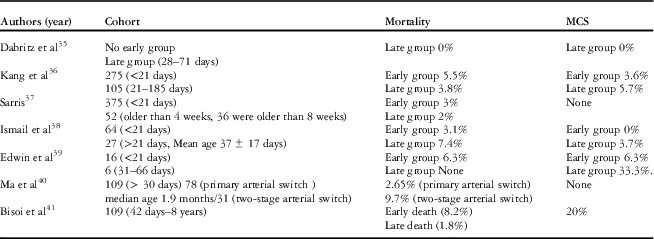
Beyond 3 months of age, most units would offer a low-morbidity atrial switch – Senning operation – with a loose pulmonary artery band for possible future conversion to an arterial switch. If the left ventricle is still prepared after 3 months, then a cardiac catheterisation is done for further evaluation. The usual causes of left ventricle preparedness beyond 3 months of age are a large patent ductus arteriosus, large aortopulmonary collaterals, left ventricular outflow tract obstruction, and pulmonary hypertension. In the event of pulmonary hypertension, an arterial switch is offered only if the pulmonary hypertension appears reversible. These infants often need perioperative management of both left ventricular dysfunction and pulmonary hypertension. In the presence of significant pulmonary vascular disease, a palliative Senning procedure with a limited atrial septal defect is a better option to relieve severe cyanosis.Reference Sharma, Choudhary and Bhan 43
Obstructed total anomalous pulmonary venous connection
Obstructed total anomalous pulmonary venous connection presenting in late infancy constitutes a surgical emergency needing emergent repair. A collapsed baby should be rushed to the operating room as soon as possible without waiting for attempts at preoperative stabilisation. Postoperatively, these infants often have profound low output owing to a non-complaint left atrium and left ventricle, as well as pulmonary hypertension. Surgical strategies to address this include leaving a decompressive patent foramen ovale in the presence of severe pulmonary hypertension and deferred sternal closure.
The postoperative low cardiac output state and pulmonary hypertension
Delayed surgery is often accompanied by severe but often transient postoperative low cardiac output state owing to systemic ventricular or biventricular dysfunction. The typical features of this have been well characterised by Wernovsky et al.Reference Wernovsky, Wypij and Jonas 44 In low- and middle-income countries, the availability of extracorporeal support and adjunctive therapies such as inhaled nitric oxide is typically very limited. Management is based on conventional multimodal practices, namely preload augmentation, appropriate inotropy, and most importantly, aggressive afterload reduction. Surgical strategies include delayed sternal closure, intraoperative placement of a peritoneal dialysis catheter in infants with anticipated low cardiac output state, and, for patients with severe pulmonary hypertension or right ventricle diastolic dysfunction, a decompressive patent foramen ovale.
Adjuvant therapies include normalising ionised calcium levels, physiologic doses of hydrocortisone, and replacement thyroxine in documented hypothyroidism. Owing to the sheer burden of late CHD, plus economic and resource constraints, great efforts are taken to avoid mechanical circulatory support. Measures to reduce metabolic demand include analgesia, sedation, muscle relaxation, and targeted temperature control. Ventilatory support, appropriate use of positive end expiratory pressure, and non-invasive ventilation are important therapies used to improve afterload in systemic ventricular dysfunction with increased end diastolic pressures.Reference Wernovsky, Wypij and Jonas 44 – Reference Bronicki and Chang 48 In high-risk patients, such as those after a delayed arterial switch operation, sternal closure, and even extubation should be carefully timed events. In principle, a common sense approach to do “only one thing at a time” – for example, waiting for a period of stability after chest closure before weaning sedation, or weaning inotropes after extubation – should be applied.
CHD-related pulmonary hypertension has been extensively reviewed elsewhere.Reference Lopes, Barst and Haworth 7 – Reference Galie, Humbert and Vachiery 9 , Reference Giannakoulas and Gatzoulis 21 , Reference Dimopoulos, Wort and Gatzoulis 22 , Reference Taylor and Laussen 49 The incidence of postoperative pulmonary hypertension is much higher in late-presenting CHD.Reference Lopes, Barst and Haworth 7 Current consensus does not favour routine use of pulmonary artery catheters or vasodilator therapy. The main focus is on avoidance of triggers of pulmonary hypertensive crises including pain, agitation, hypercarbia, acidosis, high or low lung volumes, and pre-emptive management of the low cardiac output state with milrinone. An acute crisis may be managed with inhaled nitric oxide or inhaled prostacyclin when availableReference Lopes, Barst and Haworth 7 and a single dose of sildenafil is recommended when weaning off nitric oxide.Reference Namachivayam, Theilen, Butt, Cooper, Penny and Shekerdemian 50 Alternatively, after the crisis is aborted with adequate ventilation, intravenous sildenafil or bosentan or both may be used. After pulmonary hypertensive crises, a detailed evaluation should be done to exclude residual defects causing pulmonary hypertension.
Ethical and moral dilemmas
With improved cardiac care and better economic growth, increasing numbers of neonates and infants, particularly those with late transposition of great arteries with intact interventricular septum and left-sided obstructive lesions who present in actual or pending circulatory collapse, are being offered surgery with increasing survival. However, just as for CHD globally, the importance of good-quality survival is of paramount importance, and a significant proportion of late-presenting patients in low- and middle-income countries already have pre-existing or evolving neurologic injury detectable on clinical examination or neuro-imaging before undergoing surgery. This poses an important ethical challenge in a setting where neither the family nor society can necessarily afford to take on the burden of significant neurologic disability.
Summary
We note the following conclusions or take-home messages:
-
∙ Late CHD is an increasing entity seen with burgeoning economic growth.
-
∙ Ideally, late presentation should affect a minority and not the majority, and should be eliminated as a risk factor for early surgical mortality and morbidity.
-
∙ Unambiguous guidelines for operability and management would be ideal but difficult to formulate.
-
∙ Surgery that is very likely to have adverse long-term outcomes – for example, closing a ventricular septal defect in a patient with severe pulmonary hypertension – should be avoided.
-
∙ A more conservative approach to operability in the presence of pulmonary hypertension – pulmonary vascular resistance index <6, preferably <4 – is recommended.
-
∙ Patients with polycythaemic tetralogy of Fallot can have excellent early outcomes with judicious perioperative planning.
-
∙ There is reasonable evidence to support that a primary arterial switch operation can be safely performed at up to 8 weeks of age, and potentially as late as 3 months of age if there is appropriate institutional experience and infrastructure.
-
∙ Severe low cardiac output state and/or pulmonary hypertension often complicate the postoperative course in late CHD. Attempts should be made to manage these using standard modalities and meticulous intensive care, and avoiding expensive strategies such as mechanical circulatory support or inhaled nitric oxide.
-
∙ Many practices will be validated only by midterm or long-term follow-up of survivors, with suggestions and recommendations requiring periodic review over time.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.







